Steroid substance derivation method and its analysis detection method
A detection method, sterol-based technology, applied in the field of analytical chemistry, can solve problems such as poor detection sensitivity and many side reactions, and achieve high sensitivity, convenient operation, and good derivation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
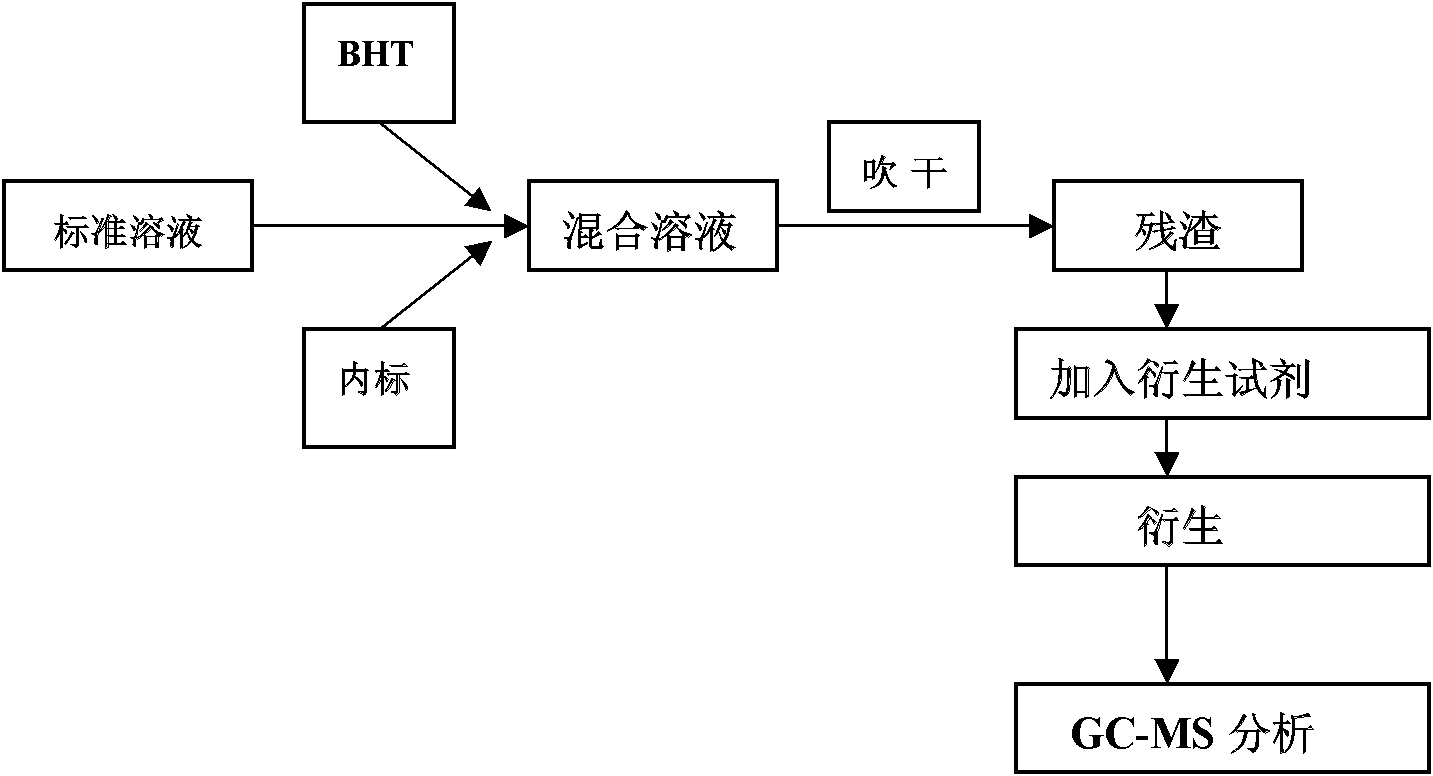
[0036] Determination of Derivation Method
[0037] (1) Preparation of reagents
[0038] 2,6-Di-tert-butyl-4-methylphenol (BHT) methanol solution: Weigh BHT powder, dissolve it in methanol, and prepare 100ng / mL BHT methanol solution, which plays an antioxidant role during the detection process.
[0039] Sterol standard substance solution: Weigh 1.5-2.0 mg of 24-dehydrocholesterol, 7-encholestanol, stigmasterol, and cholestanol standard substance respectively, dissolve them in a small amount of n-hexane, and the resulting solution Mix well, continue to add n-hexane, and prepare a series of sterol standard solutions with concentrations (between 0.001-1.400mg / dL). The normal content of non-cholesterol steroid substances in human serum is between 0.02-1.00mg / dL. The concentration selected in the technical solution of the invention covers this concentration range.
[0040] 5α-cholestane n-hexane internal standard solution: Weigh 5α-cholestane powder and dissolve it in n-hexane to ...
Embodiment 2
[0057] Derivatization reagent
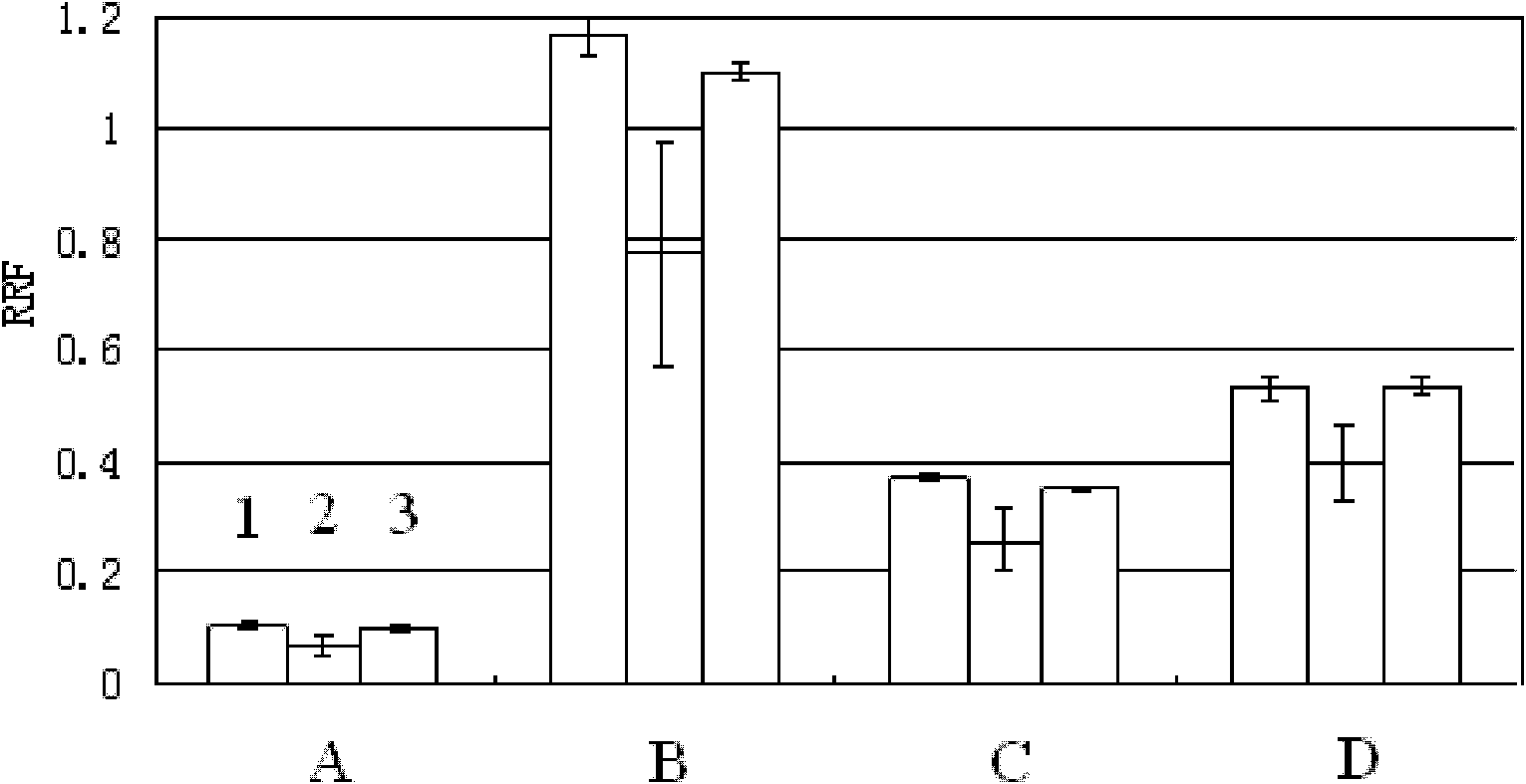
[0058] Select the derivatization reagent HMDS+TMCS+pyridine (3+1+9) and B STFA+TMCS (99+1) (volume ratio) commonly used in the derivatization of sterol substance hydroxyl group in conventional technology
[0059] Through common technical analysis, it is concluded that there is a significant difference between the derivatization effect (peak area of characteristic peak) of HMDS+TMCS+pyridine derivatization reagent and BSTFA+TMCS derivatization reagent (tfigure 2 );From figure 2 It can also be seen from Table 2 that after MSTFA derivation, the coefficient of variation of the response factor (RRF) of the quantifier ion is small (within 10%), indicating that the derivation method has good repeatability.
[0060] Table 2 The relative influence factor (RRF) of three kinds of derivatization reagents
[0061]
Embodiment 3
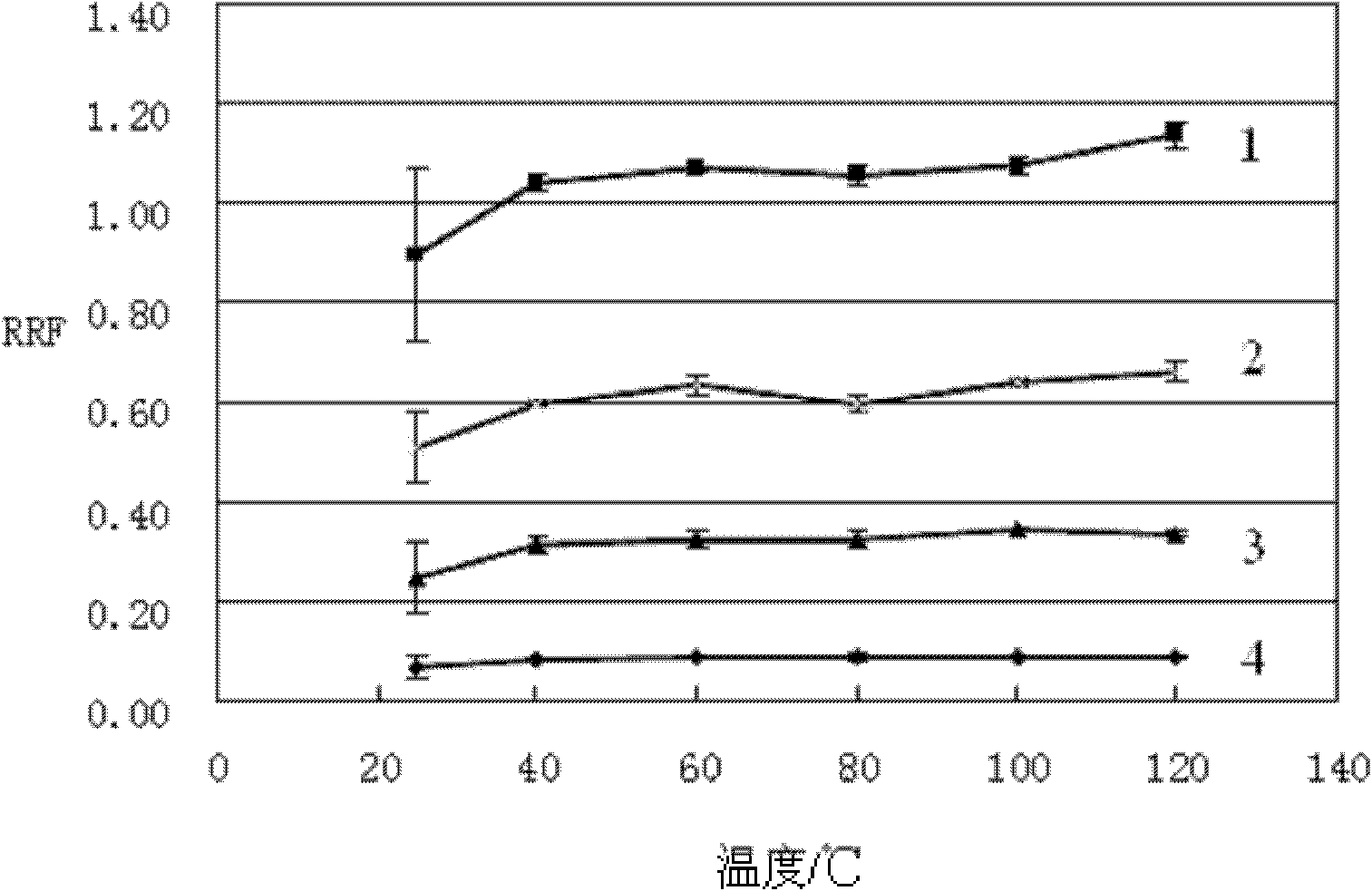
[0063] Derivation temperature
[0064] The peak area ratio ( RRF) was used as the research object to investigate the variation of RRF with derivatization temperature.
[0065] The experiment was designed to derivate at room temperature (25°C), 40°C, 60°C, 80°C, 100°C, 120°C and other temperature points, and the derivation time was uniformly adopted for 1 hour. The results are as follows image 3 shown. Above 60°C, the response factor of the quantitative ion basically does not change, so 60°C is selected as the temperature of the derivatization reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com