Anthraquinone degradation product regeneration catalyst and preparation method thereof
A technology for regenerating catalysts and anthraquinone degradation products, which is applied in physical/chemical process catalysts, quinone preparation, chemical instruments and methods, etc., can solve the problems of low regeneration efficiency, difficult separation and recovery, and the stability of aromatic tertiary amines needs to be investigated. To achieve the effect of high regeneration efficiency, saving production cost and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step A: take by weighing 54.53g Mg(NO 3 ) 2 ·6H 2 O, 89.43g hexamethylenetetramine and 0.70g SDBS were dissolved in 40mL deionized water to form a mixed solution. Wherein the molar ratio of magnesium ions to hexamethylenetetramine is 1:3, and the molar concentration of SDBS is 0.05 mol / L.
[0029] Step B: Add 20g Al 2 o 3 Carrier (spherical carrier prepared by the rolling ball method, the crystal form is γ type, the diameter is 5mm, and the specific surface area is 190m 2 g -1 , the pore volume is 0.85cm 3 / g, crushing strength is 138N / grain) was added to the mixed solution, reacted in the autoclave at 150°C for 6h, took out the sample, washed to pH=7~8, dried at 85°C for 5h to obtain hydrotalcite MgAl-CO 3 -LDHs / Al 2 o 3 Precursor, calcined at 550°C for 4h to obtain 30wt.%MgO / Al 2 o 3 Sample, wherein the mass percentage of MgO is 30wt.%.
[0030] After determination, the specific surface area of the spherical regenerated catalyst is 147m 2 / g, the pore v...
Embodiment 2
[0036] Step A: take by weighing 56.72g Be(NO 3 ) 2 ·3H 2 O, 37.93g Al(NO 3 ) 3 9H 2 O, 91.05g urea and 0.54g SDS were dissolved in 40mL deionized water to form a mixed solution. where the molar ratio Be 2+ : Al 3+ =3, the molar ratio of beryllium ions to urea is 1:5, and the molar concentration of SDS is 0.05mol / L.
[0037] Step B: Add 20g Al 2 o 3 Carrier (spherical carrier prepared by the rolling ball method, the crystal form is γ type, the diameter is 4mm, and the specific surface area is 202m 2 g -1 , the pore volume is 1.15cm 3 / g, crushing strength is 128N / grain) was added to the mixed solution, reacted in the autoclave at 120°C for 8h, took out the sample, washed to pH=7, dried at 90°C for 5h to obtain hydrotalcite BeAl-CO 3 -LDHs / Al 2 o 3 Precursor, calcined at 550°C for 4h to obtain BeO / Al 2 o 3 sample. Wherein the mass percentage of BeO is 20wt%.
[0038] It has been determined that the specific surface area of the spherical regenerated catalyst i...
Embodiment 3
[0043] Step A: take by weighing 13.01g Ca(NO 3 ) 2 4H 2 O, 13.23g Mg(NO 3 ) 2 ·6H 2 O, 21.92g urea and 0.58g CTAB were dissolved in 40mL deionized water to form a mixed solution. where the molar ratio of Ca 2+ : Mg 2+ =1:1, the molar ratio of divalent metal ions to urea is 2:7, and the molar concentration of CTAB is 0.04mol / L.
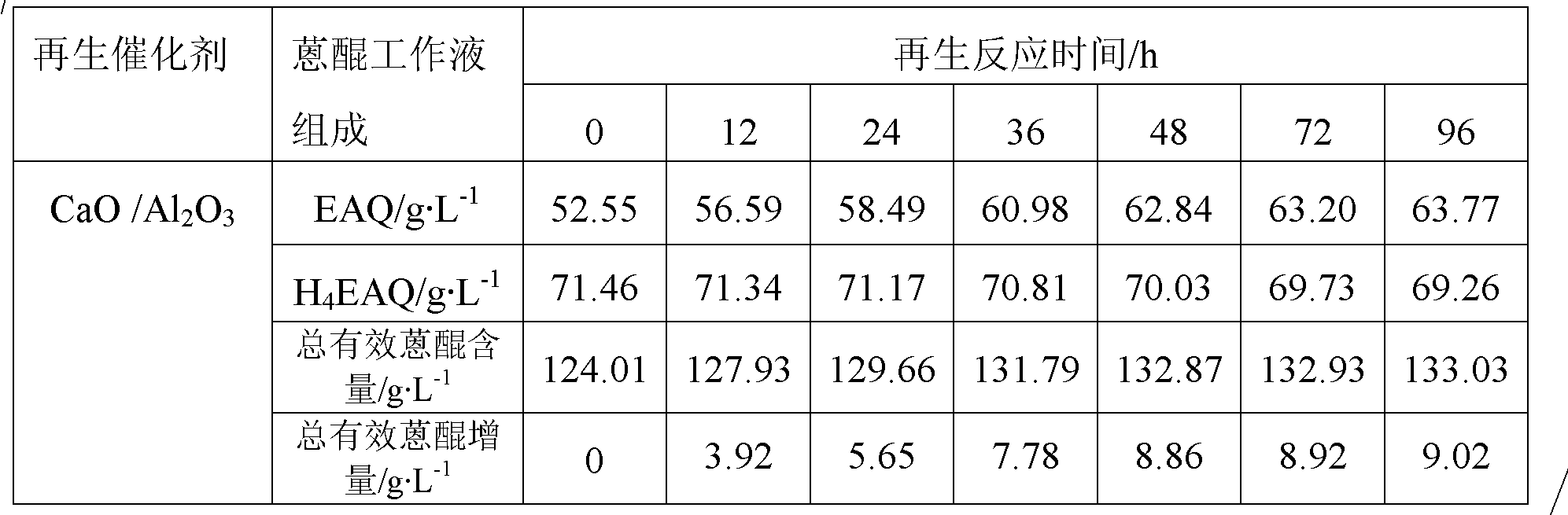
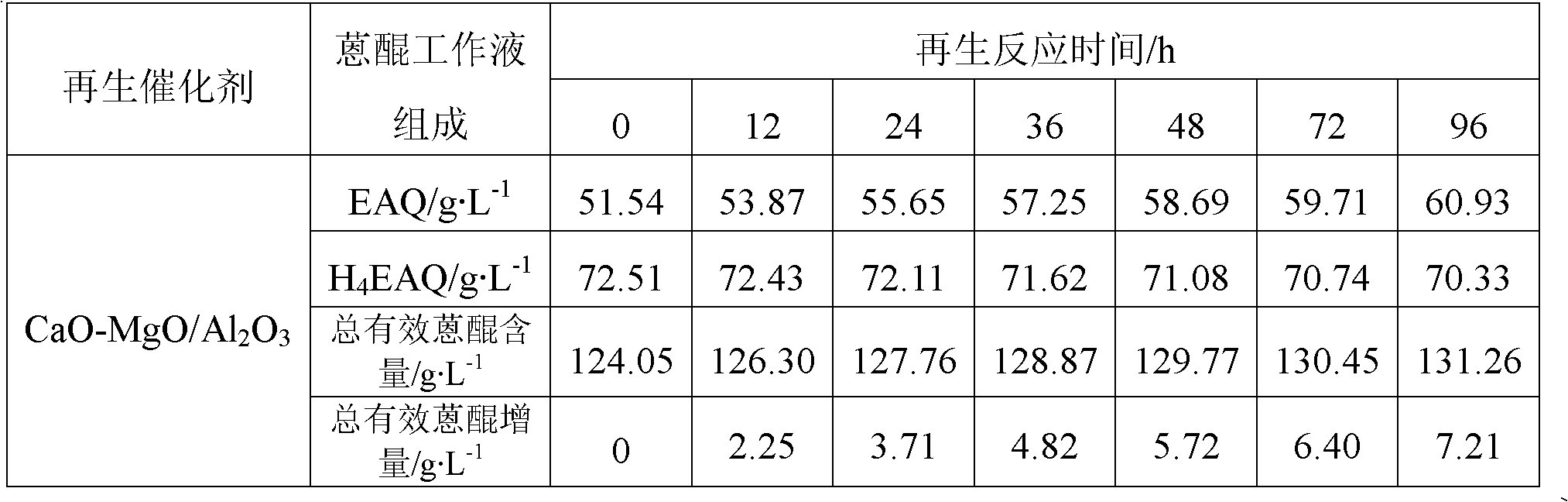
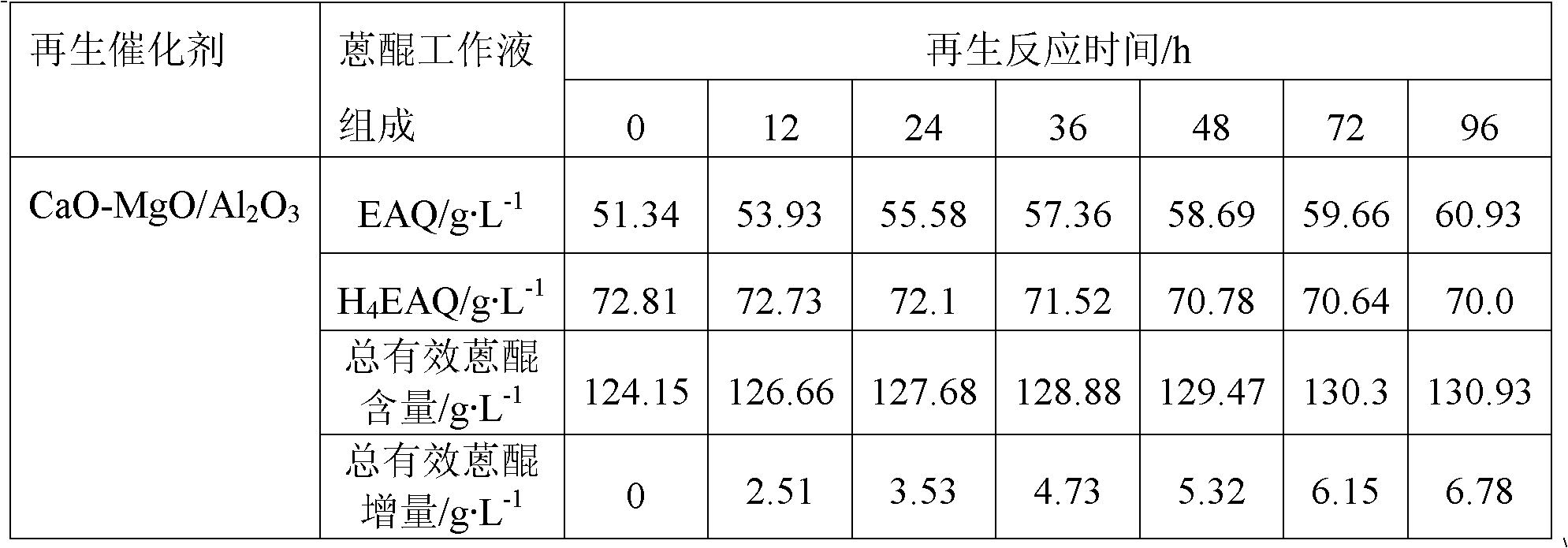
[0044] Step B: Add 20g Al 2 o 3 Carrier (a spherical carrier prepared by the oil column molding method, the crystal form is γ-type, the diameter is 2.2mm, and the specific surface area is 181m 2 g -1 , the pore volume is 1.01cm 3 / g, crushing strength is 119N / grain) was added to the mixed solution, reacted in the autoclave at 130°C for 6h, took out the sample, washed to pH=7, dried at 80°C for 5h to obtain hydrotalcite CaMgAl-CO 3 -LDHs / Al 2 o 3 Precursor, calcined at 550°C for 4h to obtain the total mass of CaO and MgO in the final product CaO-MgO / Al 2 o 3 A sample with a mass fraction of 20wt.%.
[0045] After measurement, the specif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com