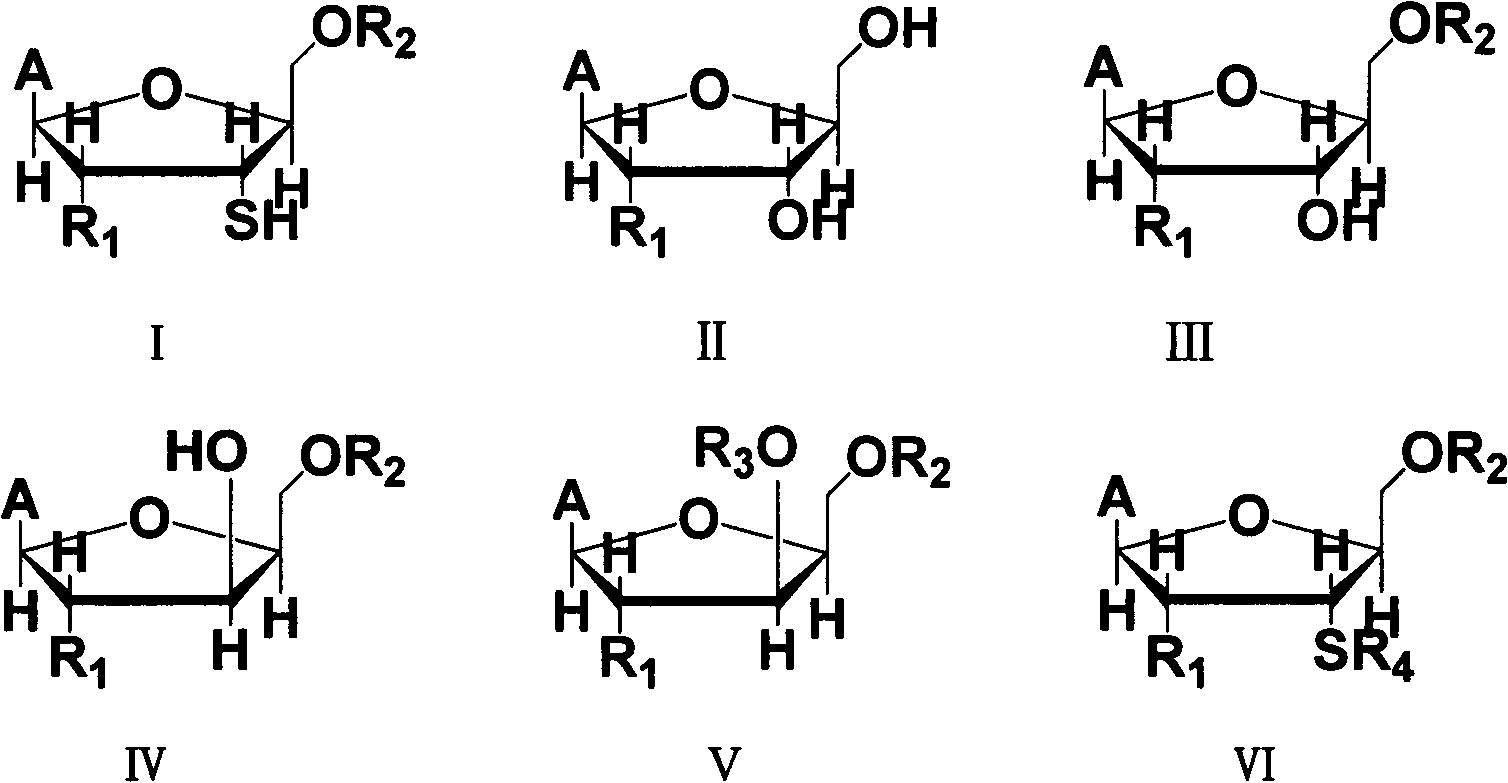
3'-thionucleoside synthesis method
A compound and mixture technology, which is applied in the production of bulk chemicals, sugar derivatives, organic chemistry, etc., can solve the problems of low purity, low yield of target products, lengthy synthetic routes, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
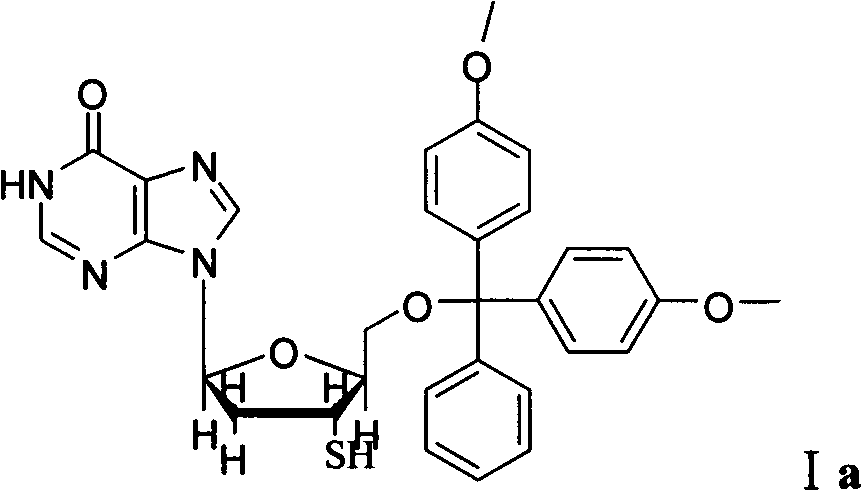
[0037] The preparation of compound shown in formula Ia:
[0038]
[0039] Dissolve 2'-deoxyinosine (0.2520g, 1.0mmol) in 10mL of treated anhydrous DBU, start stirring, and slowly add DMTCl (0.4060g, 1.2mmol) to the two-necked flask, and the reaction solution continues to be heated at room temperature. The reaction was stirred for 19h. Pass through a silica gel column, first remove pyridine with an eluent (DCM:EtOH=20:1, V / V), and then increase the polarity to obtain the product. The product is a white solid 0.5483g (5'-DMT-2'- Deoxyinosine, compound IIIa), the yield was 98.97%.
[0040] Dissolve Sareite reagent (0.6400g, 1.5mmol) in 8mL of treated anhydrous dichloromethane, start stirring, then cool in ice bath for 10min, slowly add compound 5′-DMT-2′-deoxyinosine (0.5440 g, 1.0mmol), then react in ice bath for 1h, remove the ice bath, slowly rise to room temperature and continue the reaction for 4h, add 8mL of anhydrous isopropanol dropwise to the reaction solution, add ...
Embodiment 2
[0048] The preparation of compound shown in formula I b:
[0049]
[0050] Except that 2-acetamide-2'-deoxyadenosine was substituted for 2'-deoxyinosine in Example 1, other conditions were the same as in Example 1 to obtain the title compound. The purity of the title compound was 98%, and its overall yield was about 45%.
[0051] 1 H NMR (400MHz, CDCl 3 ): δ10.32(s, 1H), 8.30(s, 1H), 6.97(s, 2H), 7.43-6.84(m, 13H), 5.94(dd, 1H, J 1 = 4.0Hz,J 2 =8Hz), 4.38(m, 1H), 3.83(s, 6H), 3.63(dd, 1H, J 1 = 4.0Hz,J 2 =8Hz), 3.38(dd,1H,J 1 = 4.0Hz,J 2 =8Hz), 2.90(m, 1H), 2.68(m, 1H), 2.43(m, 1H), 2.04(s, 3H), 1.58(d, 1H, J=8Hz).
[0052] MS(ESI): m / z calcd for C 33 h 34 N 6 o 5 S: [M+H + ]: 627.2; found: 627.3.
Embodiment 3
[0054] The preparation of compound shown in formula Ic:
[0055]
[0056] According to the method described in Example 1, only changing the raw material 2′-deoxyinosine to 2-acetamide-6-benzamide-2′-deoxyadenosine, and DMTCl to TBSCl can successfully obtain the title compound. The purity of the title compound was 98%, and its overall yield was about 49%.
[0057] 1 H NMR (400MHz, CDCl 3 ): δ11.34(s, 1H), 10.32(s, 1H), 8.36(s, 1H), 8.05-6.84(m, 5H), 5.94(dd, 1H, J 1 = 4.0Hz,J 2 =8Hz), 4.38(m, 1H), 4.20(m, 1H), 3.73(s, 1H), 2.93(dd, 1H, J 1 = 4.0Hz,J 2 =8Hz), 2.68(dd,1H,J 1 = 4.0Hz,J 2 =8Hz), 2.40(m, 1H), 2.08(s, 3H), 1.60(d, 1H, J=8Hz), 0.21(s, 9H).
[0058] MS(ESI): m / z calcd for C 22 h 28 N 6 o 4 SSi: [M+H + ]: 501.2; found: 502.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com