A kind of preparation method of thiadiazole derivative
A technology of thiadiazole derivatives and dimercaptothiadiazole, which is applied in lubricating compositions, organic chemistry, additives, etc., can solve the problems of long reaction time, catalyst separation, and high proportion of by-products, and achieve simple process and low by-product less, faster response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The invention provides a preparation method of thiadiazole derivatives, comprising:
[0032] S1, using active metal as catalyst, adding dimercaptothiadiazole and primary hydrogen peroxide in water-soluble solvent, reacting to generate dimercaptothiadiazole dimer; wherein, preferably, active metal is capable of strong specific adsorption with DMTD Metal element; It is further preferred that the active metal is elemental copper; including:
[0033] S11, using elemental copper as a catalyst, adding ethanol or ethanol aqueous solution as a water-soluble solvent in the reactor, adding dimercaptothiadiazole (DMTD) and a catalyst in the water-soluble solvent, and stirring evenly; wherein:
[0034] The catalyst used in the present invention is simple copper, and after the catalyst is used, it can be used for the next reaction, or it can be taken out after cleaning for subsequent use;
[0035] The water-soluble solvent used in the present invention is ethanol or an aqueous solu...
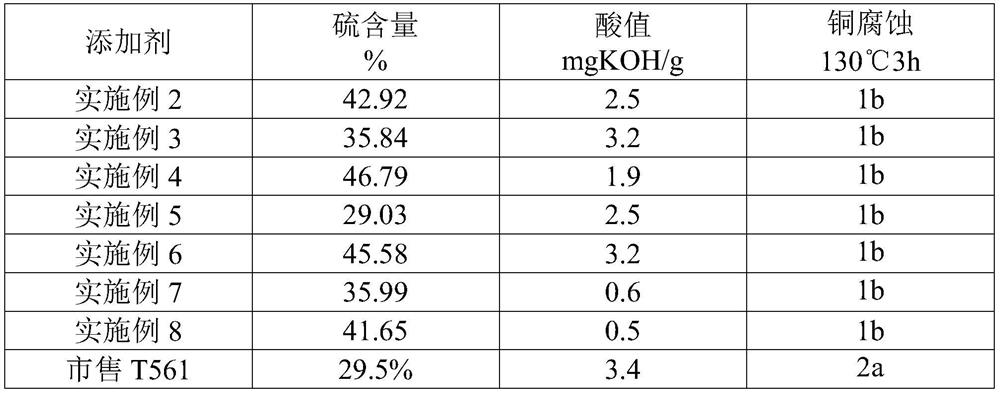
Embodiment 1
[0059] In a clean three-neck flask with stirring and heating and cooling devices, add 10g of dimercaptothiadiazole, 40g of ethanol, and put 10 non-oxidized copper sheets (total area: 10mm 2 ), turn on stirring. After fully stirring for 10 minutes, the temperature was raised to 50° C., 8.32 g of hydrogen peroxide (30%) was added dropwise, and the reaction was kept for 30 minutes. The copper sheet was taken out from the reaction system to obtain a mixed system of DMTD dimer intermediate and solvent, which was directly used in subsequent reactions without separation.
Embodiment 2
[0061] Using the mixed solution of DMTD dimer and solvent obtained by the method in Example 1, 10.17 g of nonyl mercaptan was added at one time, and the temperature was raised to 75°C after thorough stirring. 11.33 grams of hydrogen peroxide (30%) was added dropwise, and the system temperature was not greater than 77° C. during the dropwise addition. After the dropwise addition was completed, react at 75° C. for 1.5 h.
[0062] Stand to separate the liquid, remove the lower organic phase, wash the organic phase 3 times with 15ml of hot water at 95°C, and distill off the moisture in the organic phase under reduced pressure at 105°C and 0.09MPa vacuum to obtain the thiadiazole derivative of the invention thing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com