Novel organic photoconductor molecule and synthetic method thereof
An organic photoconductor and synthesis method technology, applied in the preparation of organic compounds, organic chemistry, semiconductor devices, etc., can solve the problems of high cost, uneconomical practicality, insufficient coverage, etc. The effect of the synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Compound (1) N, the synthesis of N'-two (p-ethylphenyl)-N, N'-two (p-hydroxyphenyl) dibenzidine:
[0049] Synthetic roadmap:
[0050]
[0051] 333 g p-ethylaniline, 320 ml 4-methoxybromobenzene, 9 g palladium acetate, 45 g 1,1′-bis(diphenylphosphino)ferrocene (DPPF) and 336 g sodium tert-butyloxide Dissolve in 6.5 liters of dry toluene. The mixture was heated and stirred at 90° C. for 20 hours under the protection of nitrogen. The reaction was monitored by TLC until complete. After filtering and washing with petroleum ether, 530 g of brown product 4-ethyl-N-(p-methoxyphenyl)aniline was obtained. Yield: 93%.
[0052] Second step reaction:
[0053]
[0054] 300 grams of 4-ethyl-N-(p-methoxyphenyl)aniline, 202 grams of 4,,4'-dibromobiphenyl, 7 grams of palladium acetate, 35 grams of 1,1'-bis(diphenylphosphine ) ferrocene (DPPF) and 190 grams of sodium tert-butylate were dissolved in 3 liters of dry toluene. The mixture was heated and stirred at 9...
Embodiment 2
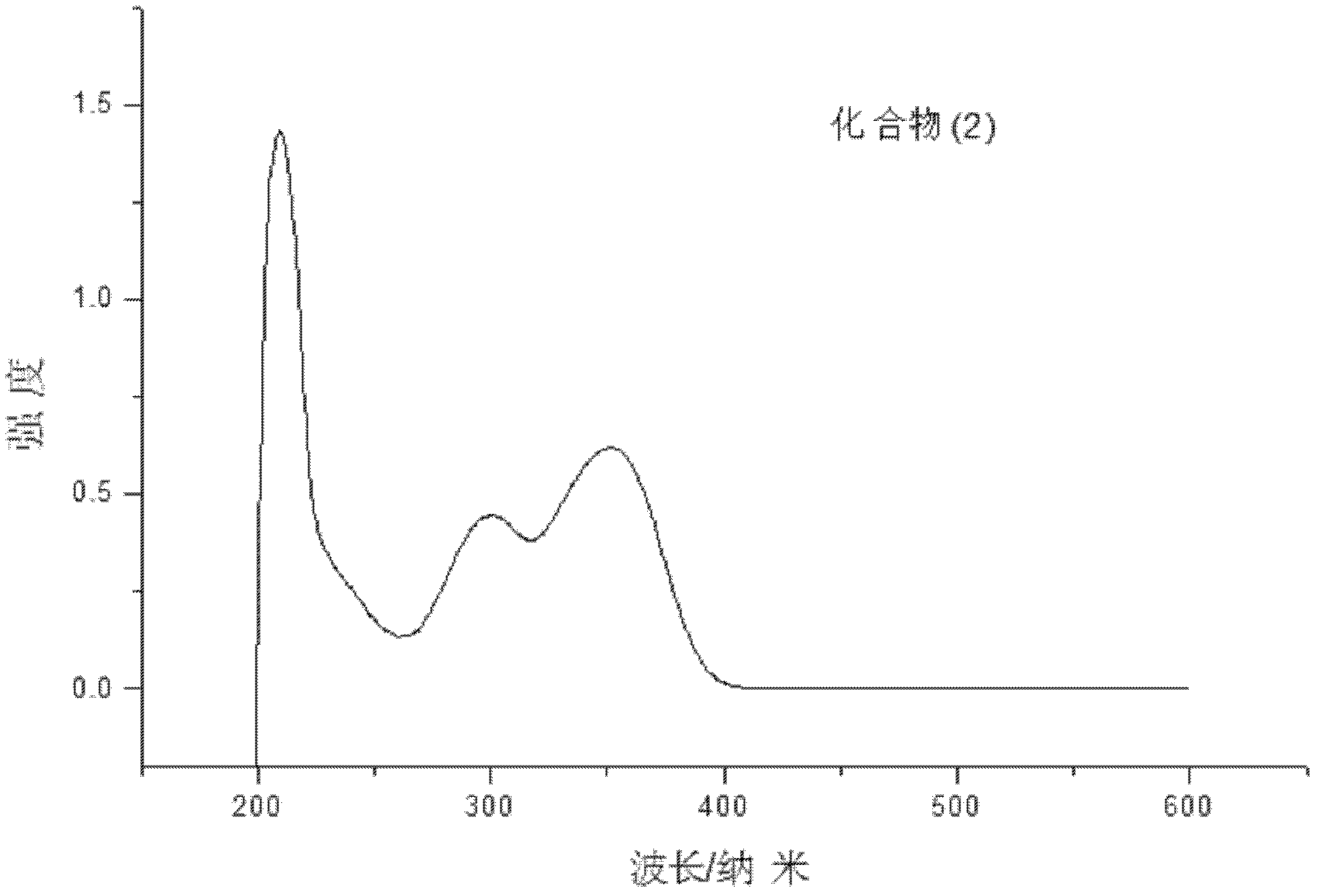
[0058] Embodiment 2: Compound (2) N, the synthesis of N'-two (p-isopropylphenyl)-N, N'-two (p-hydroxyphenyl) dibenzidine:
[0059] Synthetic roadmap:
[0060]
[0061] First step response:
[0062]
[0063] 150 g p-cymene, 128 ml 4-methoxybromobenzene, 3.6 g palladium acetate, 18 g 1,1′-bis(diphenylphosphino)ferrocene (DPPF) and 135 g tert-butyl alcohol Sodium was dissolved in 3 liters of dry toluene. The mixture was heated and stirred at 90° C. for 20 hours under the protection of nitrogen. The reaction was monitored by TLC until complete. After filtering and washing with petroleum ether, 215 grams of brown product 4-isopropyl-N-(p-methoxyphenyl)aniline were obtained. Yield: 89%.
[0064] Second step reaction:
[0065]
[0066] 215 grams of 4-isopropyl-N-(p-methoxyphenyl)aniline, 133 grams of 4,,4'-dibromobiphenyl, 4.8 grams of palladium acetate, 23.8 grams of 1,1'-bis(diphenyl Phosphino)ferrocene (DPPF) and 124 grams of sodium tert-butylate were dissolved in ...
Embodiment 3
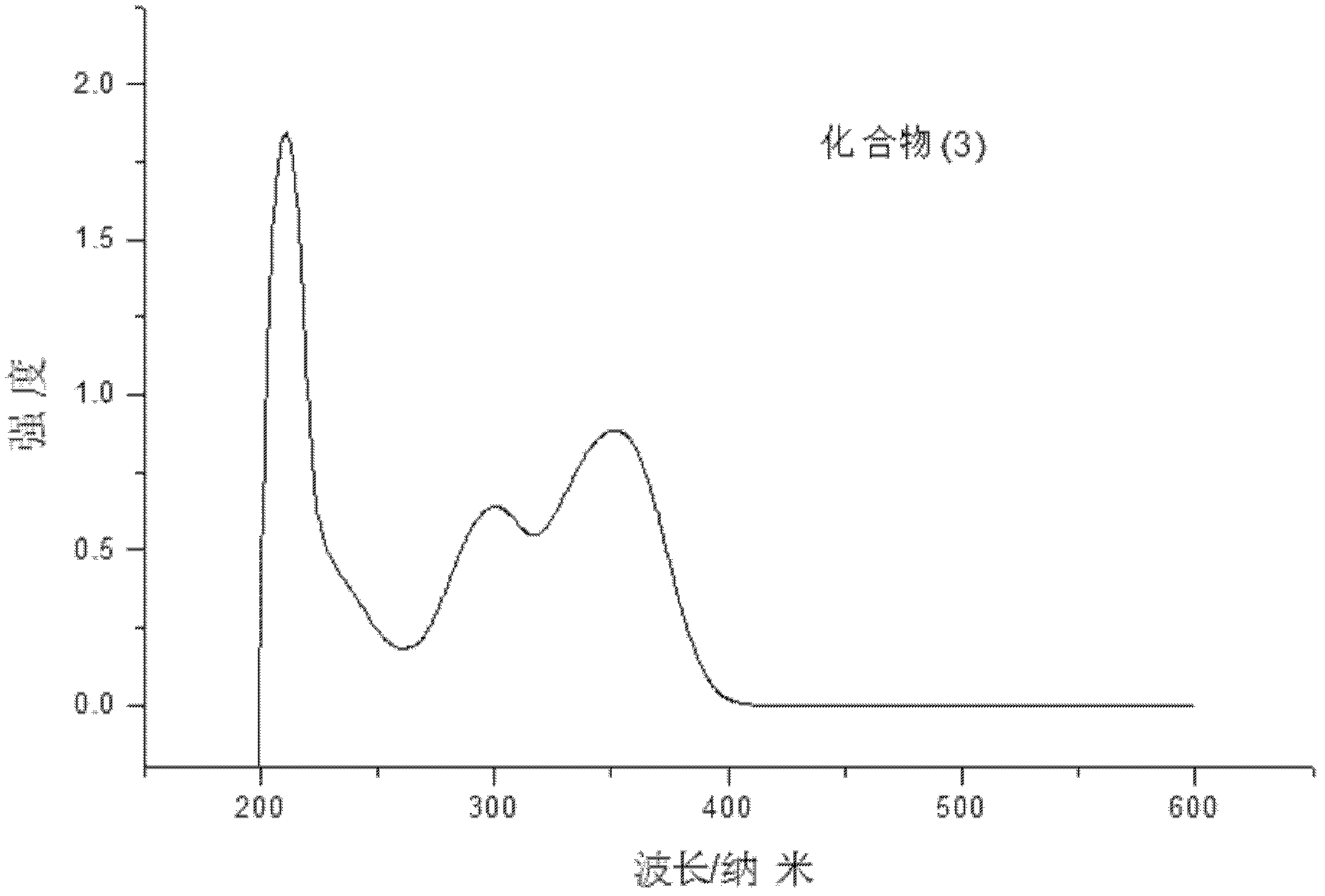
[0070] Embodiment 3: Compound (3) N, the synthesis of N'-bis(p-ethylphenyl)-N,N'-bis(p-hydroxymethylphenyl)dianidine: synthetic route map:
[0071]
[0072] 500 g of p-bromobenzyl alcohol were dissolved in 3.5 liters of dichloromethane. After the dropwise addition of 419 g of tert-butyldimethylsilyl chloride, the solution was cooled to 0°C. Then 316 ml of pyridine was added dropwise, keeping the solution temperature at 0°C for at least 20 minutes. Afterwards, the ice bath was removed and the reaction was stirred at room temperature for 5 hours. Monitoring by TLC showed that the reaction of raw material p-bromobenzyl alcohol was complete. Then the mixture was washed with 5% hydrochloric acid (2x1L) and water (2x1L), and dried over anhydrous magnesium sulfate. Thereafter, the solvent was distilled off to obtain 810 g of p-bromobenzyloxy-tert-butyldimethylsilane as an oily product. Yield: 100%.
[0073] Second step reaction:
[0074]
[0075] 340 grams of p-ethylanili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com