Method for preparing sulfur ether intermediates of proton pump inhibitor
A thioether and proton pump technology, applied to thioether intermediates 2-[S-arylmethyl]thio-1H-benzimidazole compound, 2-[S-arylmethyl]thio-1H-imidazo In the field of [4,5-b]pyridine compounds, it can solve the problems of unfavorable cost and cumbersome operation, and achieve the effects of avoiding oxidative deterioration, simple reaction steps and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
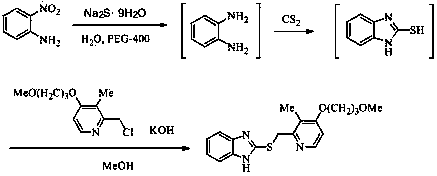
[0065] Proton pump inhibitor temoprazole sulfide intermediate 2-[ S -(pyridin-2-yl)methyl]thio-1 H - the preparation of benzimidazole
[0066]
[0067] 150 mL of water was added to the reaction flask, and 2-nitroaniline (13.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 4 hours, then cooled the reaction system to 40 °C, directly added CS without separation 2 (9.0 mL, 0.15 mol), after continuing to reflux for 7 hours, add 70 mL methanol, 2-chloromethylpyridine (15.3 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol) , heated to reflux again, and reacted for 4 hours. The reaction solution was evaporated under reduced pressure to remove methanol, poured into 100 mL of ice water, a large amount of solids were precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 15.3 g of light yellow solid, yield 64.6%, mp 105~108 ℃.
[0068] Spectral data: EI-MS ( m...
Embodiment 2
[0071] Proton pump inhibitor Lansoprazole sulfide intermediate 2-[ S -(3-Methyl-4-(2,2,2-trifluoro)ethoxypyridin-2-yl)methyl]thio-1 H - the preparation of benzimidazole
[0072]
[0073] 150 mL of water was added to the reaction flask, and 2-nitroaniline (13.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 4 hours, then cooled the reaction system to 40 °C, directly added CS without separation 2 (9.0 mL, 0.15 mol), after continuing to reflux for 7 hours, add 70 mL methanol, 2-chloromethyl-3-methyl-4-(2,2,2-trifluoro)ethoxy Basepyridine (28.8 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol), heated to reflux again, and reacted for 4 hours. The reaction solution was evaporated under reduced pressure to remove methanol, poured into 100 mL of ice water, a large amount of solids precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 26.5 g of light yellow so...
Embodiment 3
[0078] 150 mL of water was added to the reaction flask, and 2-nitro-4-methoxyaniline (16.8 g, 0.1 mol), Na 2 S·9H 2 O (72 g, 0.3 mol) and PEG-400 (2.0 mL), heated to reflux, reacted for 5 hours, then cooled the reaction system to 40 °C, directly added CS 2 (9.0 mL, 0.15 mol), after continuing to reflux for 5.5 hours, 70 mL of methanol, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine (22.3 g, 0.12 mol), KOH (13.2 g, 85%, 0.2 mol), heated to reflux again, and reacted for 4 hours. The reaction liquid was evaporated to remove methanol under reduced pressure, poured into 100 mL of ice water, a large amount of solids precipitated, and the yellow crude product was obtained by suction filtration, which was recrystallized with petroleum ether to obtain 23.7 g of light yellow solid, yield 71.9%, mp 118~119 ℃.
[0079] Spectral data: EI-MS ( m / z ): 329 [M + ];
[0080] 1 H-NMR (400 MHz, CDCl 3 , ppm), 8.21 (1H, s, pyridine), 7.42 (1H, d, J = 8.8 Hz, ArH), 7.10 (1H, d, J = 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com