Organic electroluminescent material of large-pi conjugated system and preparation method and application thereof
A technology of electroluminescent materials and conjugated systems, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., to achieve the effects of improved efficiency, simple synthesis, and good film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
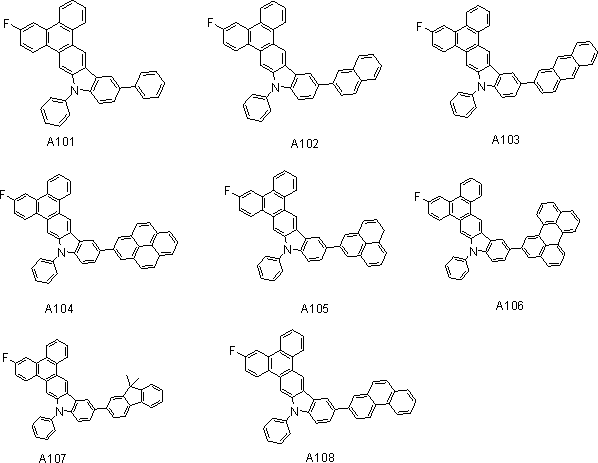
[0019] Example 1 Synthesis of Type A Compound
[0020] Synthesis of compound A101
[0021]
[0022] Under a nitrogen protection system, weigh 100mmol (15.6g) of aryl halide bromobenzene and 110mmol (50g) of arylboronic acid into the reaction system, add 440mmol (46.64g) of anhydrous Na2CO3 solid, and add 220ml of water to the system. Added 440ml of toluene, under the protection of nitrogen, the catalyst Pd(PPh3) 4 5mmol (5.77g), react at a constant temperature of 90℃ under the protection of nitrogen for 8-12 hours, after the reaction is cooled, filtered with suction, washed, extracted, concentrated, dichloromethane As a solvent, silica gel was used for column chromatography and concentrated to obtain 43.85 g of an off-white final product with a yield of over 85% and an HPLC purity of over 98%.
[0023] Mass spectrum: calculated value is 487.17; tested value is 487.15. Elemental analysis: calculated value C: 88.68%; H: 4.55%; F: 3.70%; N: 2.87%; test value: C: 88.66%; H: 4.52%; F: 3...
Embodiment 2
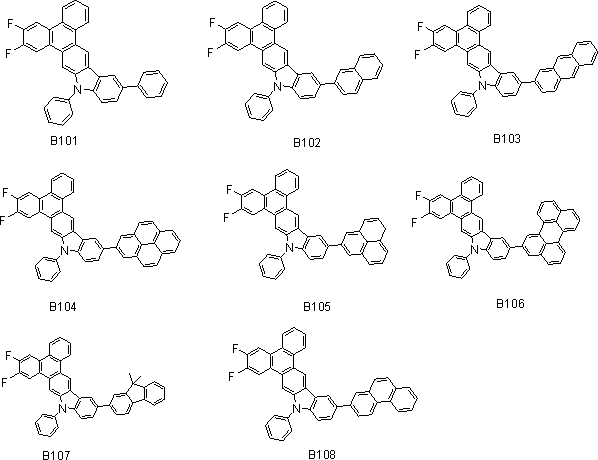
[0040] Example 2 Synthesis of Type B Compound
[0041] Synthesis of B101
[0042]
[0043] Under a nitrogen protection system, weigh 100mmol (15.6g) of aryl halide bromobenzene and 110mmol (52g) of arylboronic acid into the reaction system, add 440mmol (46.64g) of anhydrous Na2CO3 solid, and add 220ml of water to the system. Added 440ml of toluene, under the protection of nitrogen, the catalyst Pd(PPh3) 4 5mmol (5.77g), react at a constant temperature of 90℃ under the protection of nitrogen for 8-12 hours, after the reaction is cooled, filtered with suction, washed, extracted, concentrated, dichloromethane As a solvent, silica gel was used for column chromatography and concentrated to obtain 43.69 g of an off-white final product with a yield of over 85% and an HPLC purity of over 98%.
[0044] Mass spectrum: calculated value is 505.16; tested value is 505.15. Elemental analysis: calculated value C: 85.53%; H: 4.19%; F: 7.52%; N: 2.77%; test value: C: 85.50%; H: 4.17%; F: 7.50%; N: ...
Embodiment 3
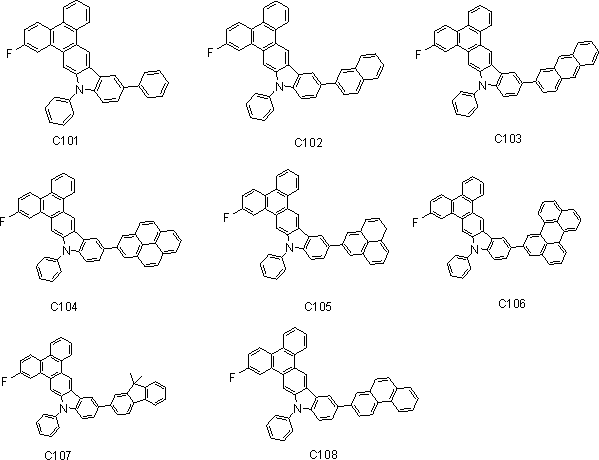
[0061] Example 3 Synthesis of Type C Compound
[0062] Synthesis of compound C101
[0063]
[0064] Under a nitrogen protection system, weigh 100mmol (15.6g) of aryl halide bromobenzene and 110mmol (50g) of arylboronic acid into the reaction system, add 440mmol (46.64g) of anhydrous Na2CO3 solid, and add 220ml of water to the system. Added 440ml of toluene, under the protection of nitrogen, the catalyst Pd(PPh3) 4 5mmol (5.77g), react at a constant temperature of 90℃ under the protection of nitrogen for 8-12 hours, after the reaction is cooled, filtered with suction, washed, extracted, concentrated, dichloromethane As a solvent, silica gel was used for column chromatography and concentrated to obtain 42.86 g of the off-white final product, the yield was over 85%, and the HPLC purity was over 98%.
[0065] Mass spectrum: calculated value is 487.17; tested value is 487.15. Elemental analysis: calculated value C: 88.68%; H: 4.55%; F: 3.90%; N: 2.87%; test value: C: 88.66%; H: 4.52%; F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com