Novel intermediate for synthesizing silodosin as well as preparation method and purpose of novel intermediate
A technology for compounds and enantiomers, applied in the field of preparation of intermediate compounds, can solve the problems of high cost and complicated preparation process, and achieve the effects of reducing production cost, controllable optical purity, and avoiding by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
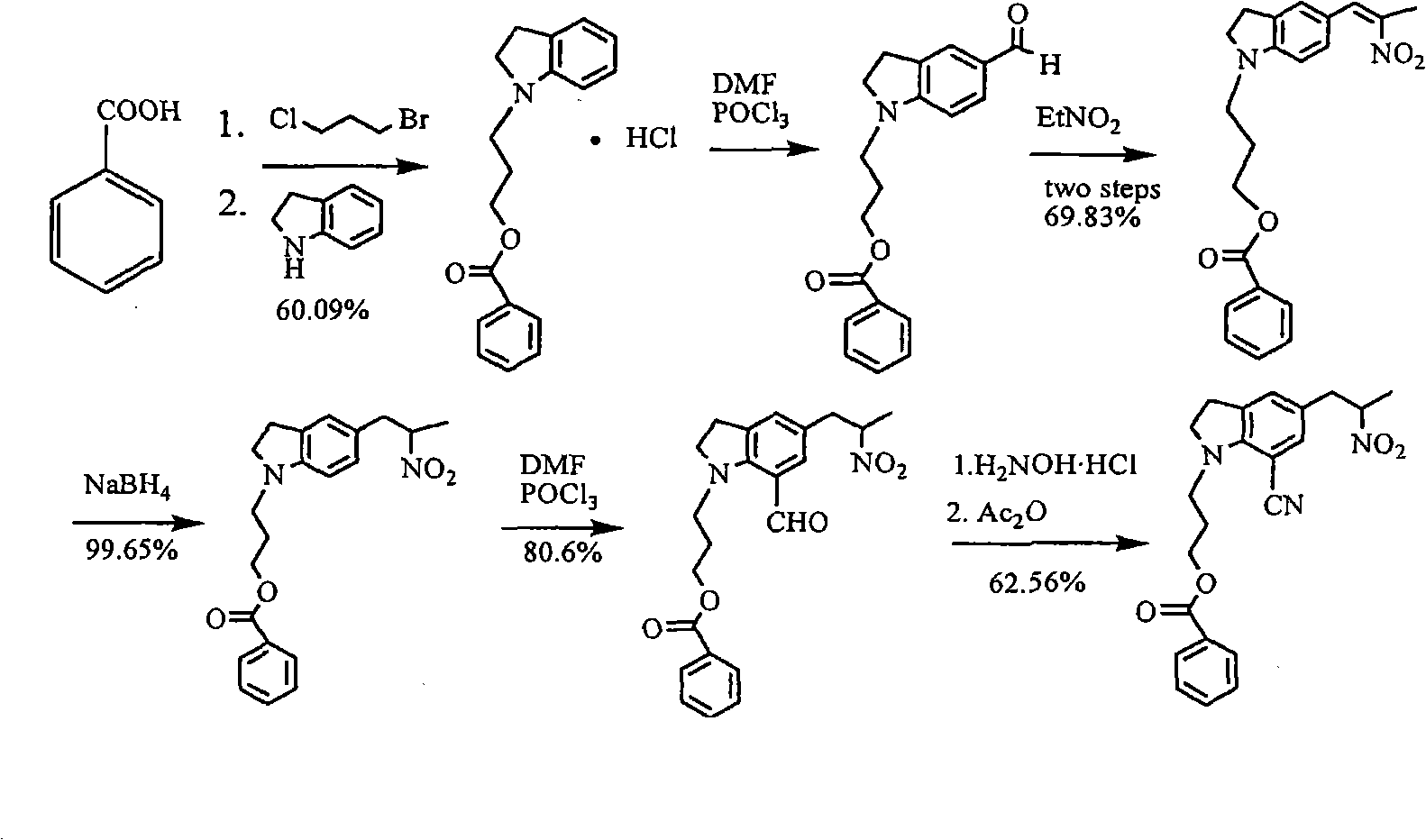
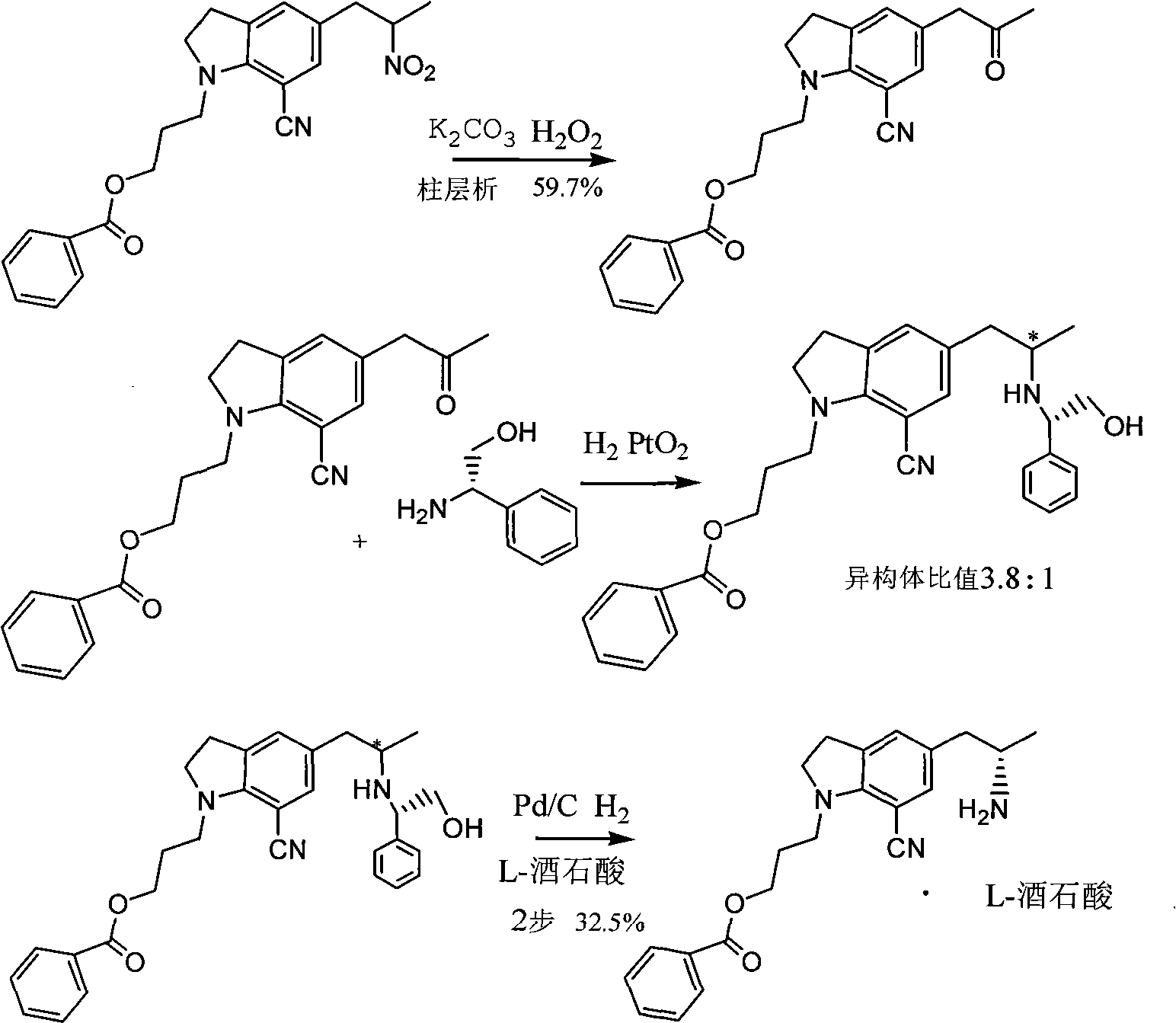
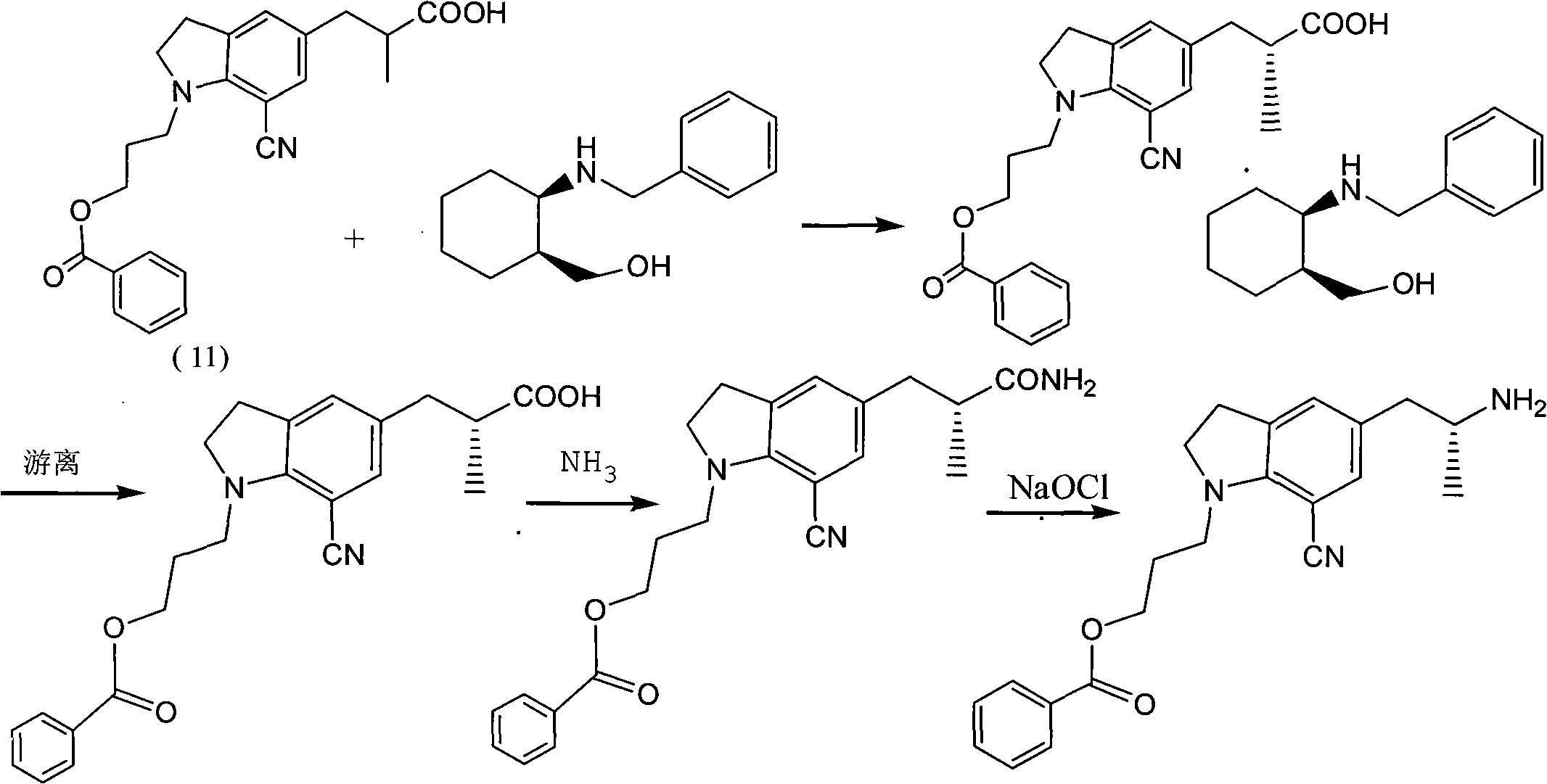
[0032] Example 1 Preparation of 5-[2-(amino)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanindoline L-tartrate
[0033] Add 3 kg of methanol into the hydrogenation kettle, then add 152.4 g of glacial acetic acid, 500 g of 5-[2-(nitro)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanoindol Indoline, 50g of 10% palladium on carbon, after nitrogen replacement, heat to 60-65°C, feed hydrogen to 4-5 atmospheric pressure, react for 12 hours, monitor the reaction by TLC, after the reaction is completed, cool to room temperature, filter to remove palladium on carbon, a little methanol Rinse the palladium carbon, the filtrate is concentrated under reduced pressure and evaporated to remove the solvent, under stirring, add to the potassium carbonate solution prepared by 525.7g of potassium carbonate and 2 kg of water, add 1500 ml of tetrahydrofuran, separate the layers, and add 209.7g of the organic phase The solution prepared by L-tartaric acid and 1258 grams of water was cooled to about zero degree and ...
Embodiment 2 6
[0037] The preparation of embodiment two to six 5-[2-(amino)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanoindoline L-tartrate
[0038] Add methanol to the hydrogenation kettle, then add glacial acetic acid, 5-[2-(nitro)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanindoline, 10 % palladium carbon, after nitrogen replacement, heat to 60-65°C, feed hydrogen to 4-5 atmospheric pressure, react for 12 hours, TLC monitors the reaction, after the reaction is completed, cool to room temperature, remove palladium carbon by filtration, and rinse the palladium carbon with a little methanol , the filtrate was concentrated under reduced pressure and evaporated to remove the solvent, and then added to the prepared aqueous potassium carbonate solution under stirring, then extracted with tetrahydrofuran, separated, the organic phase was added to the pre-prepared L-tartaric acid aqueous solution, cooled to about zero, and kept for 3 Hours, the solid was filtered and weighed after drying.
[0039] The speci...
Embodiment 2 6
[0040] Table 1 embodiment two to six preparation 5-[2-(amino) propyl]-1-[3-(benzoyloxy) propyl]-7-cyanoindoline L-tartrate feed amount and results
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com