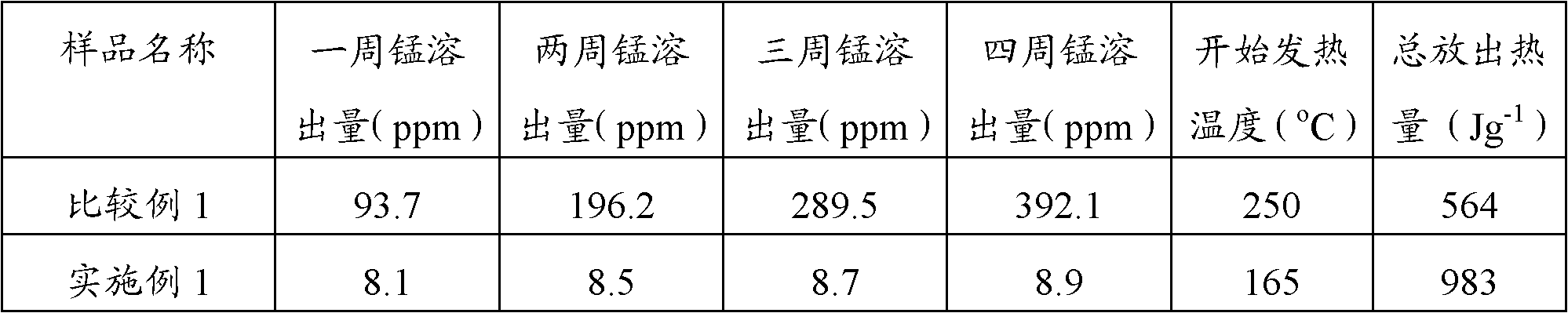
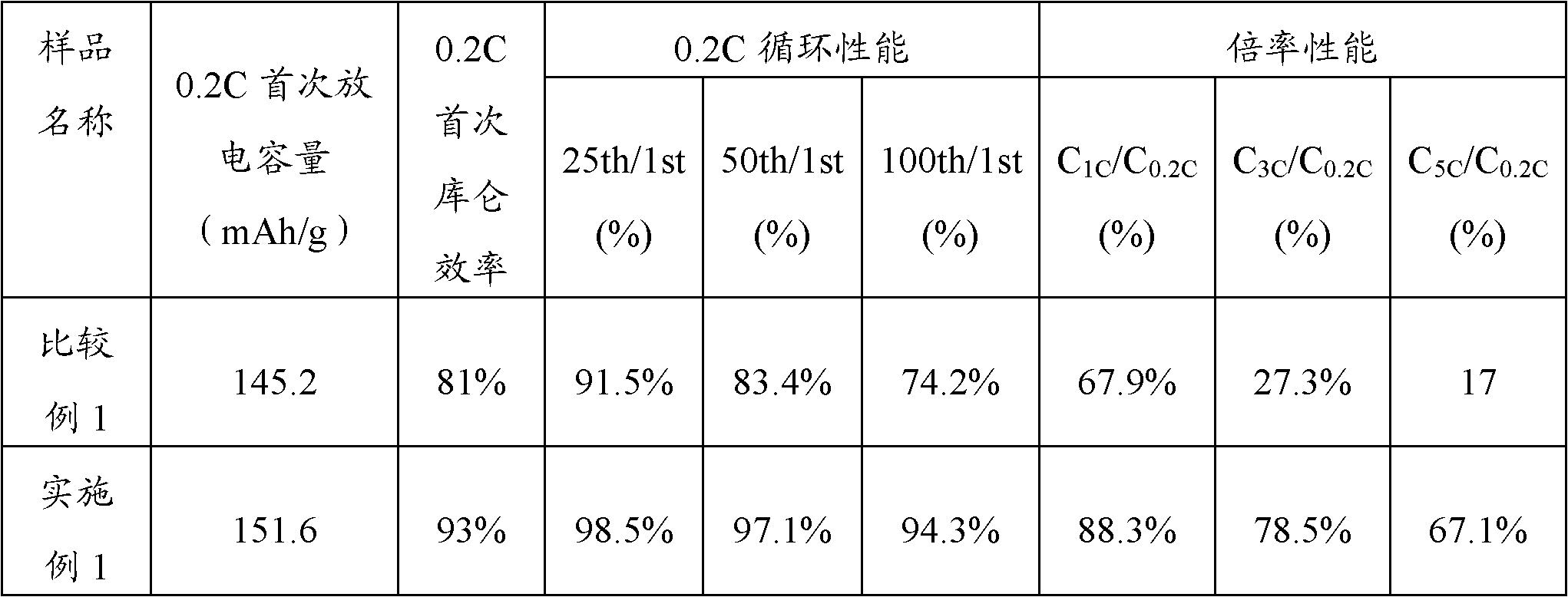
Lithium manganese phosphate positive pole material and preparation method thereof
A cathode material, lithium manganese phosphate technology, used in battery electrodes, electrical components, circuits, etc., can solve problems such as not being able to achieve good improvement, reduce manganese dissolution, and achieve suppression of lattice distortion and good surface structure. Effects of stability, guaranteed safety performance and cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention discloses a preparation method of lithium manganese phosphate cathode material, which comprises the following steps:
[0025] Step a) mix the aqueous solution of Mn source compound and M1 source compound with PO 4 3- Mix in the reaction kettle, adjust the pH value to 2-7, and get Mn after reaction 1-y-z Ml y PO 4 , the concentration of the Mn source compound in the aqueous solution is 1-y-z mol / L, the concentration of the M1 source compound in the aqueous solution is y mol / L, 0≤y≤0.3, 0<z≤0.3;
[0026] Step b) Add the aqueous solution of the M2 source compound dropwise to the reaction kettle, adjust the pH value to 2-7, and the M2 source compound and PO 4 3- reaction to form Mn coated with phosphate of M2 1-y-z Ml y PO 4 , the concentration of the aqueous solution of the M2 source compound is z mol / L;
[0027] Step c) mixing the product obtained in step b), the lithium source compound and the carbon source compound, ball milling and dry mixing, roas...
Embodiment 1
[0038](1) Dissolve 0.7mol of manganese nitrate and 0.05mol of iron nitrate (M1 compound) in 1L of deionized water to form a mixed solution containing Mn and Fe elements, wherein the concentration of Mn is 0.7mol / L, and the concentration of Fe is 0.05 mol / L; Dissolve 0.25mol of ferric nitrate (M2 compound) in 1L of deionized water to form a solution containing Fe element, wherein the concentration of Fe is 0.25mol / L; dilute 85% concentrated phosphoric acid with deionized water to obtain 1mol / L phosphoric acid solution 1L; prepare 1mol / L NH 3 ·H 2 O solution 1L;
[0039] (2) Use a peristaltic pump to slowly drop the mixed solution containing Mn and Fe elements prepared above and the phosphoric acid solution into the transmission reactor at the same time, wherein the drip rate of the peristaltic pump is 0.1mL / s, and the heating temperature of the reactor is 30 ℃, the stirring speed is 100r / min, by adjusting the NH 3 ·H 2 The drop rate of the O solution stabilizes the pH valu...
Embodiment 2
[0042] (1) Dissolve 0.8mol of manganese carbonate and 0.1mol of magnesium sulfate (M1 compound) in 1L of deionized water to form a mixed solution containing Mn and Mg elements, wherein the concentration of Mn is 0.8mol / L, and the concentration of Mg is 0.1 mol / L; Dissolve 0.1mol of cobalt sulfate (M2 compound) in 1L of deionized water to form a solution containing Co element, wherein the concentration of Co is 0.1mol / L; dilute 85% concentrated phosphoric acid with deionized water to obtain 1mol / L phosphoric acid solution 1L; prepare 1mol / L NH 3 ·H 2 O solution 1L;
[0043] (2) Use a peristaltic pump to slowly drop the mixed solution containing Mn and Mg elements and the phosphoric acid solution into the transmission reactor at the same time, wherein the dripping rate of the peristaltic pump is 0.5mL / s, and the heating temperature of the reactor is 50 ° C. The speed is 500r / min, by adjusting NH 3 ·H 2 The drop rate of the O solution stabilizes the pH value of the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com