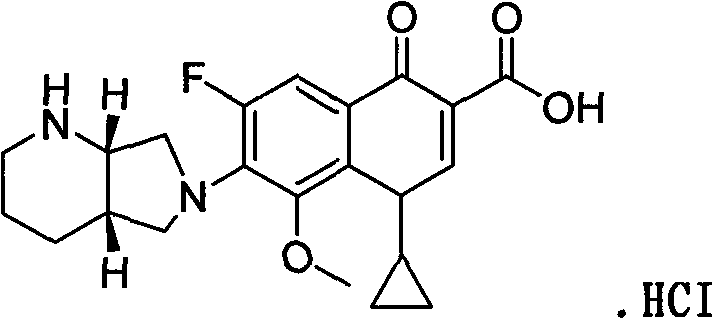
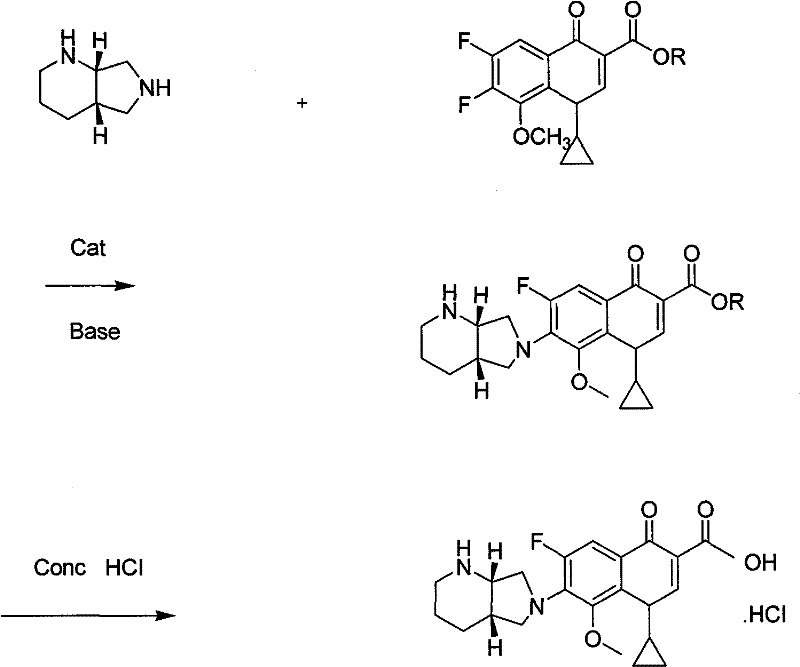
Preparing method of moxifloxacin or slat thereof
A technology of star ester and cyclopropyl, which is applied in the field of medicinal chemistry, can solve the problems of many preparation steps, low yield, and low product yield, and achieve the effects of simple and easy-to-control process, low manufacturing cost, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 700 milliliters of ethanol, add 32.3 grams of 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester and 13.2 grams S, S-2,8-diazabicyclo[4.3.0]nonane, add 0.4 gram of anhydrous aluminum chloride catalyst, stir, add 2.5 gram of triethylamine, stir reaction at 45 ℃ of temperature control, TLC (thin layer Chromatography) to judge the reaction to the end point, cool slightly, adjust the pH value to 2-3 with 35% to 37% concentrated hydrochloric acid, control the temperature at 70°C and stir the reaction for 2 to 3 hours, cool to below 10°C to crystallize, filter, and vacuum dry The obtained product-moxifloxacin hydrochloride was 39.8 grams, with a yield of 91% and a purity of 99.2%.
Embodiment 2
[0034] Get 1300 milliliters of methanol, add 61.8 grams of 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid methyl ester and 26.4 grams S, S-2,8-diazabicyclo[4.3.0]nonane, add 0.4 g of anhydrous aluminum chloride / 0.8 g of zinc chloride catalyst, stir, add 5.0 g of triethylamine, stir at 45°C Reaction, TLC judged that the reaction reached the end point, cooled slightly, adjusted the pH value to 2~3 with 35%~37% concentrated hydrochloric acid, controlled the temperature at 70°C and stirred for 2 to 3 hours, cooled to below 10°C to crystallize, filtered, vacuum The product was dried to obtain 78.0 grams of moxifloxacin hydrochloride, with a yield of 89.0% and a purity of 99.1%.
Embodiment 3
[0036] Take 700 milliliters of ethanol, add 32.3 grams of 1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester and 12.5 grams S, S-2,8-diazabicyclo[4.3.0]nonane, add 0.4 g of anhydrous aluminum chloride catalyst, stir, add 2.5 g of triethylamine, stir at 45°C for reaction, TLC judges the reaction to At the end point, cool slightly, use 35% to 37% concentrated hydrochloric acid to adjust the pH value to 2 to 3, control the temperature at 70°C and stir for two to three hours, cool to below 10°C to crystallize, filter, and vacuum dry to obtain the product - molybdenum hydrochloride Cifloxacin 39.5 g, yield 90.2%, purity 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com