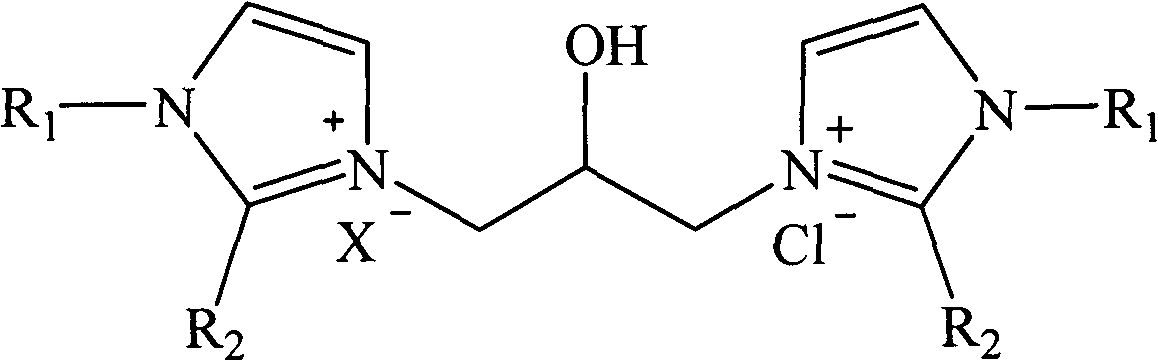
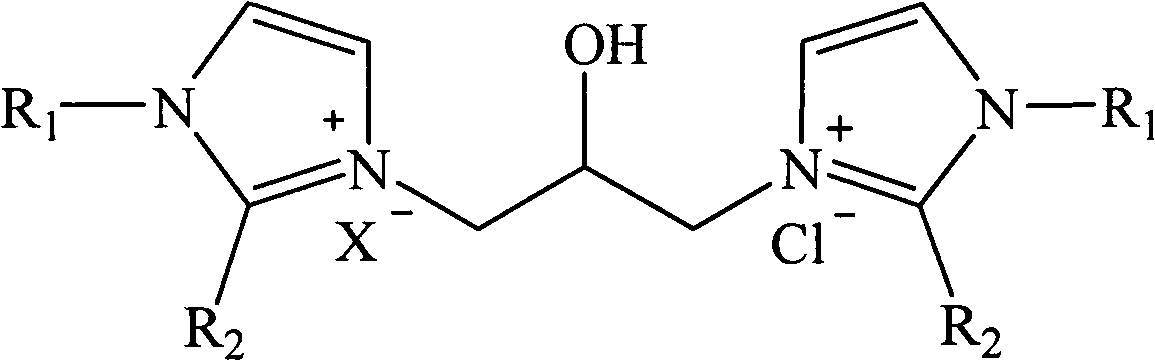
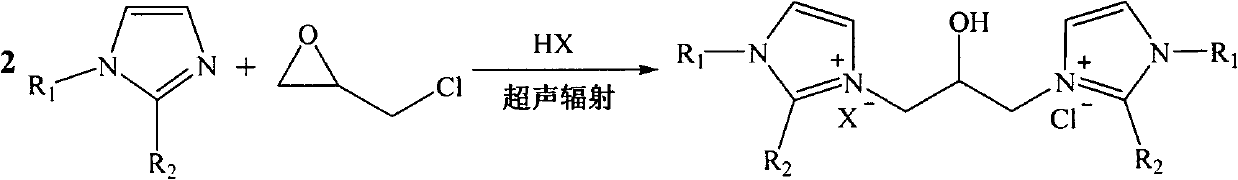
Hydroxyl-containing bivalent imidazole type ionic liquid, preparation method thereof, and application thereof
A hydroxyl-containing divalent imidazole-type and hydroxyl-divalent imidazole-type technology is applied in the field of hydroxyl-containing divalent imidazole-type ionic liquids and their preparations, which can solve the problems of ionic liquid loss of cellulose, thermal and chemical instability, etc., and achieve electrical High chemical stability, good solubility, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 : Preparation of 1,3-bis-(1-alkylimidazolyl)-2-propanol hydrochloride ionic liquid
[0027] Dissolve 40mL of 1-methylimidazole (20.6g, 0.50mol) in 80mL of ethanol, and add 21mL of concentrated hydrochloric acid (0.25mol) in batches under stirring at room temperature. -Chloro-propylene oxide (23.12g, 0.25mol) was added dropwise to the reaction solution. After the drying tube was equipped, the reaction bottle was placed in the water bath of the experimental ultrasonic cleaner, and the reaction was performed under ultrasonic radiation (power 300W, frequency 40KHz) at 30°C for 2.5h. The solvent was removed by rotary evaporation at 60°C under reduced pressure, and a colorless liquid (59.33 g, 83%) was obtained after vacuum drying. 1 H-NMR (500MHz, D 2 O): δ8.47(s, 1H), 8.39(s, 1H), 7.30(m, 4H), 4.22(dd, J=2.10, 13.20Hz, 1H), 4.20(m, 2H), 3.66(s , 3H), 3.70(s, 3H), 3.45(m, 2H). 13 C-NMR (500MHz, D 2 O): δ136.34(CH), 134.82(CH), 123.44(CH), 122.78(CH), 122.69(C...
Embodiment 2
[0028] Example 2 : Preparation of 1,3-bis-(1-alkylimidazolyl)-2-propanol hydrochloride tetrafluoroborate ionic liquid
[0029] Dissolve 40mL of 1-methylimidazole (20.6g, 0.50mol) in 90mL of ethanol, add 33mL of tetrafluoroboric acid (0.25mol) in batches under stirring at room temperature, after the addition, the reaction solution is naturally cooled to room temperature, and 19.6mL of 3-Chloro-propylene oxide (23.12 g, 0.25 mol) was added dropwise to the reaction solution. After the drying tube was equipped, the reaction bottle was placed in the water bath of the ultrasonic cleaner used in the experiment, and was subjected to ultrasonic radiation (power 300W, frequency 40KHz) at 40°C for 1.5h. The solvent was removed by rotary evaporation at 60°C under reduced pressure, and a light yellow liquid (71.48 g, 83%) was obtained after vacuum drying. 1 H-NMR (500MHz, D 2 O): δ8.58(s, 1H), 8.43(s, 1H), 7.30(m, 4H), 4.29(dd, J=2.25, 13.25Hz, 1H), 4.127(m, 2H), 3.74(s , 3H), 3.73(s,...
Embodiment 3
[0030] Example 3 : Preparation of 1,3-bis-(1-alkylimidazolyl)-2-propanol hydrochloride acetate ionic liquid
[0031] Dissolve 40mL of 1-methylimidazole (20.6g, 0.50mol) in 90mL of ethanol, and add 14mL of acetic acid (0.25mol) in batches under stirring at room temperature. - Propylene oxide (23.12 g, 0.25 mol) was added dropwise to the reaction solution. After the drying tube was equipped, the reaction bottle was placed in the water bath of the ultrasonic cleaner used in the experiment, and reacted under ultrasonic radiation (power 300W, frequency 40KHz) at 25°C for 3.5h. The solvent was removed by rotary evaporation at 60°C under reduced pressure, and a colorless liquid (68.11 g, 86%) was obtained after vacuum drying. 1 H-NMR (500MHz, D 2 O): δ8.62(s, 1H), 8.48(s, 1H), 7.36(m, 4H), 4.34(dd, J=2.36, 13.40Hz, 1H), 4.22(m, 2H), 3.84(s , 3H), 3.76(s, 3H), 3.60(m, 2H), 2.22(s, 3H). 13 C-NMR (500MHz, D 2 O): δ188.14(C=O), 138.88(CH), 136.24(CH), 125.44(CH), 124.48(CH), 123.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com