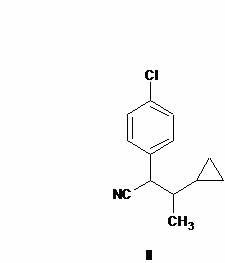
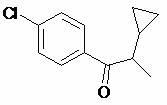
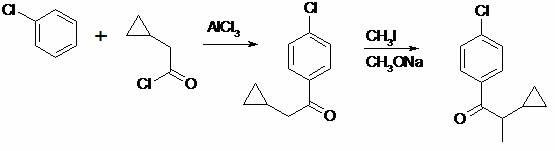
Preparation method of cyproconazole key intermediate 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A technology of cyproconazole and chlorophenyl, which is applied in the field of preparation of the key intermediate 1--2-cyclopropyl-1-propanone of cyproconazole, can solve the problem of high cost, low total yield, Three wastes and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of compound (II):
[0026] Add 44 grams of 2-(4-chlorophenyl)-3-cyclopropyl-2-butenenitrile into a 1000 ml autoclave, then add 200 grams of methanol, 1.5 grams of 5% palladium-carbon alloy, and use Replace the air with nitrogen three times, and replace it with hydrogen twice to maintain a hydrogen pressure of 1.2MPa, then slowly heat to 60 degrees and react for about 8 hours, take a sample and analyze the reaction is complete, cool, release the pressure, filter and recover the catalyst after the material is released, and the filtrate is first normal pressure The methanol was recovered by distillation, and the methanol was thoroughly distilled under reduced pressure to obtain about 41.9 grams of a viscous substance with a purity of about 95% by GC analysis. Purified to obtain 42.0 g with a molar yield of 95.4%. In actual production, no further purification is required, and it is directly used in the next reaction.
Embodiment 2
[0028] Synthesis of compound (I);
[0029] Add 22 grams of compound (II) to 66 grams of dry DMSO, then add 22 grams of potassium hydroxide powder, stir evenly, and slowly and uniformly introduce oxygen flow. At this time, the reaction will exotherm, and the internal temperature will be kept at about 40 degrees. Introduce oxygen for about 16 hours, take a sample and analyze the reaction is complete, cool to 20 degrees, add 200 ml of water to dilute the reaction solution, then neutralize it with 10% dilute hydrochloric acid, extract twice with 100 ml of toluene, and combine the extracted The toluene liquid was washed once with water, and the toluene was recovered by atmospheric distillation first, and then the toluene was thoroughly distilled under negative pressure, and the obtained crude product was distilled by high vacuum with an oil pump, and about 17.5 grams of 95% (GC, area normalization method) purity of 1 -(4-Chlorophenyl)-2-cyclopropyl-1-acetonate (I), pale yellow liqu...
Embodiment 3
[0031] Synthesis of compound (II):
[0032] Add 44 grams of 2-(4-chlorophenyl)-3-cyclopropyl-2-butenenitrile into a 1000 ml autoclave, then add 200 grams of methanol, 1.5 grams of 5% palladium-carbon alloy, and use Replace the air with nitrogen three times, and replace it with hydrogen twice to maintain a hydrogen pressure of 1.2MPa, then slowly heat to 60 degrees and react for about 8 hours, take a sample and analyze the reaction is complete, cool, release the pressure, filter and recover the catalyst after the material is released, and the filtrate is first normal pressure The methanol was recovered by distillation, and the methanol was thoroughly distilled under reduced pressure to obtain about 42.4 grams of a viscous substance with a purity of about 95% by GC analysis. Purified to obtain 41.8 g with a molar yield of 95.0%. In actual production, no further purification is required, and it is directly used in the next reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com