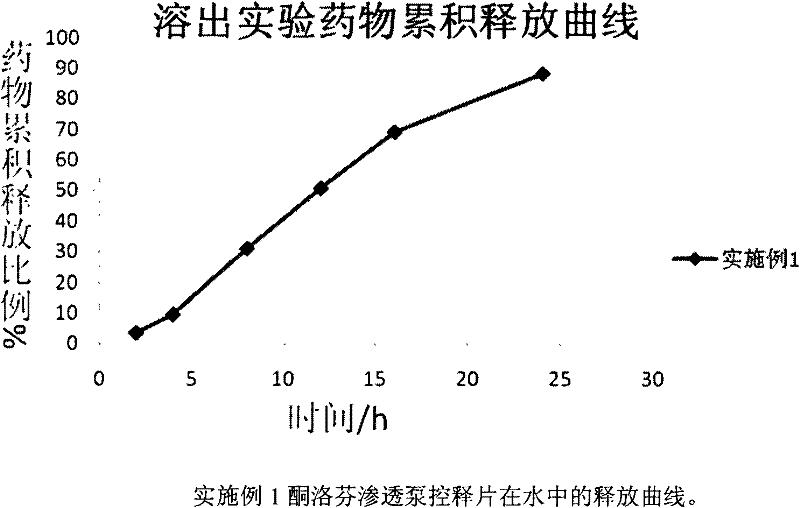
Ketoprofen osmotic pump type controlled release preparation and preparation method thereof
A technology of controlled-release preparations and osmotic pumps, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of poor patient compliance, large fluctuations in blood concentration of ordinary oral preparations, and unfavorable clinical treatment. Stable curative effect, reduced medication frequency, and good drug release correlation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Tablet Core Prescription (1000 Tablets)
[0027]
[0028] Prescription of semi-permeable membrane coating solution:
[0029]
[0030] Preparation Process:
[0031] Pass the medicine and auxiliary materials except the lubricant through an 80-mesh sieve, fully mix, add magnesium stearate, talcum powder, micropowder silica gel, mix evenly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate, pore-forming agent PEG400, and plasticizer diethyl phthalate in acetone as a coating liquid, put the tablet core in a coating pan, and coat at a coating temperature of 40-50°C, and the coating is completed Afterwards, it was cured in a drying oven at 40°C for 12 hours. Then, a drug release hole with a diameter of 1.0 mm is prepared by laser drilling on one side of the coated tablet to obtain a ketoprofen monolayer osmotic pump controlled release tablet.
Embodiment 2
[0033] Tablet Core Prescription (1000 Tablets)
[0034]
[0035] Prescription of semi-permeable membrane coating solution:
[0036]
[0037] Preparation Process:
[0038] Pass the medicine and auxiliary materials except the lubricant through an 80-mesh sieve, fully mix, add magnesium stearate, talcum powder, micropowder silica gel, mix evenly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate, pore-forming agent PEG400, and plasticizer diethyl phthalate in acetone as a coating liquid, put the tablet core in a coating pan, and coat at a coating temperature of 40-50°C, and the coating is completed Afterwards, it was cured in a drying oven at 40°C for 12 hours. Then, a drug release hole with a diameter of 1.0 mm is prepared by laser drilling on one side of the coated tablet to obtain a ketoprofen monolayer osmotic pump controlled release tablet.
Embodiment 3
[0040] Tablet Core Prescription (1000 Tablets)
[0041]
[0042] Prescription of semi-permeable membrane coating solution:
[0043]
[0044] Preparation Process:
[0045] Pass the medicine and auxiliary materials except the lubricant through an 80-mesh sieve, fully mix, add magnesium stearate, talcum powder, micropowder silica gel, mix evenly, and directly compress into tablets to obtain tablet cores. Dissolve cellulose acetate, pore-forming agent PEG400, and plasticizer diethyl phthalate in acetone as a coating liquid, put the tablet core in a coating pan, and coat at a coating temperature of 40-50°C, and the coating is completed Afterwards, it was cured in a drying oven at 40°C for 12 hours. Then, a drug release hole with a diameter of 1.0 mm is prepared by laser drilling on one side of the coated tablet to obtain a ketoprofen monolayer osmotic pump controlled release tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com