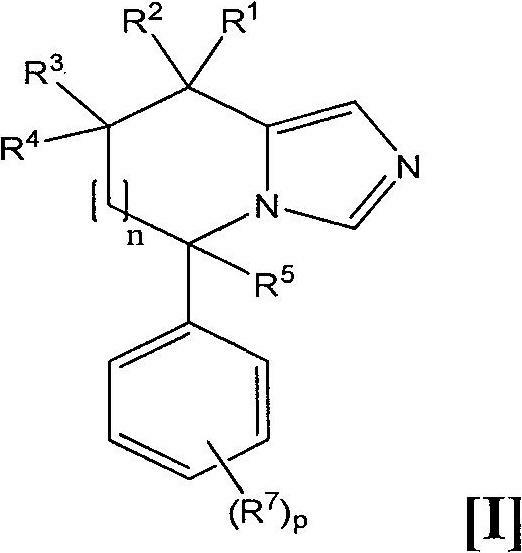
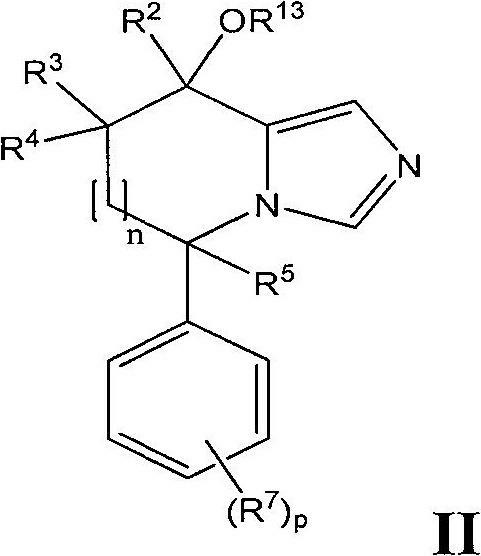
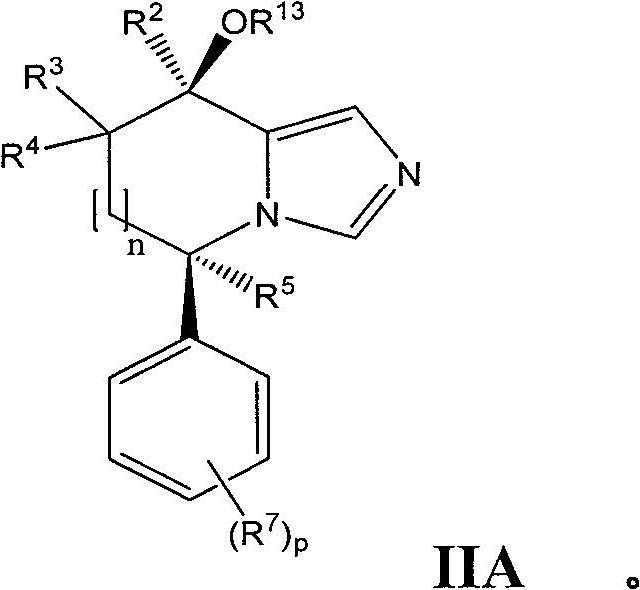
Imidazole derivatives as aldosterone synthase inhibitors
Technology of a compound, alkyl, applied in the field of imidazole derivatives as aldosterone synthase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0350] Example 1-1: 3-fluoro-4-((5R,7S)-7-hydroxy-7-vinyl-6,7-dihydro-5H-pyrrolo[1,2-c]imidazole-5- base) benzonitrile
[0351]
[0352] Stirred 3-fluoro-4-((R)-7-oxo-6,7-dihydro-5H-pyrrolo[1,2-c]imidazole-5- To a solution of benzonitrile (0.6 g, 2.5 mmol) in THF (20 mL) was added via syringe a solution of vinylmagnesium bromide (0.7 M) in THF (4.3 mL, 3.0 mmol). The crude product was warmed to room temperature over 3 hrs. use NH 4 Aq. Cl quenched the crude product. The crude product was diluted with EtOAc. with MgSO 4 The organic layer was dried, filtered and concentrated. By preparing plates, using 5% (2M NH 3 MeOH solution) / CH 2 Cl 2Purify the crude product twice and then use RPHPLC using 10%-40% MeCN / H 2 O was purified to give 142 mg of the title product as the major diastereomer. MS 270.2 (M+H); LCMS condition A, retention time 0.99min. 1H NMR (400MHz, CDCl 3 -d) δppm 2.56(br.s., 1H), 2.68(dd, 1H), 3.22(dd, 1H), 5.24(d, 1H), 5.41(d, 1H), 5.59-5.73(m, 1H) ...
Embodiment 1-2
[0354] Example 1-2: 4-((5R,7S)-7-(3-cyclopropylphenyl)-7-hydroxyl-6,7-dihydro-5H-pyrrolo[1,2-c]imidazole -5-yl)-3-fluorobenzonitrile
[0355]
[0356] To a solution of 1-bromo-3-cyclopropyl-benzene (0.018ml, 0.124mmol) in THF (0.2ml) cooled to -78°C was added 1.6M t-BuLi (0.155ml, 0.249mmol). The crude product was stirred at -78 °C for 2 hrs. To this solution was added 3-fluoro-4-((R)-7-oxo-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl)-benzonitrile (20 mg , 0.083 mmol) in THF (0.2 ml). The crude product was stirred at -78°C for 1 hr, then at 0°C for 1 hr, and at room temperature for 1 hr. The reaction was quenched with water and CH 2 Cl 2 Aqueous extraction. use Na 2 SO 4 The organic layer was dried, filtered and concentrated. By preparing plates, using 10% (2M NH 3 MeOH solution) / CH 2 Cl 2 The crude product was purified. The title product (4 mg) was isolated as the major diastereomer. MS 360.1 (M+H); LCMS condition B, retention time 1.16min. 1H NMR (400MHz, CD...
Embodiment 1
[0358] Example 1: 34-((5R,7S)-7-(4-(1,1-difluoroethyl)phenyl)-7-hydroxyl-6,7-dihydro-5H-pyrrolo[1, 2-c]imidazol-5-yl)-3-fluorobenzonitrile
[0359]
[0360] To a solution of 1-(1,1-difluoro-ethyl)-4-iodo-benzene (167 mg, 0.62 mmol) in THF (2 mL) cooled to -40 °C was added dropwise a 2M solution of i-PrMgCl in THF ( 0.42 mL, 0.83 mmol). The crude product was stirred at -40°C for 1 hr. Add 3-fluoro-4-((R)-7-oxo-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl)-benzonitrile (125 mg, 0.52 mmol) solution in THF (2 mL). The crude product was slowly warmed to room temperature over 3 hrs. use NH 4 Aq. Cl quenched the crude product. The crude product was diluted with EtOAc. The organic layer was washed with brine, MgSO 4 Dry, filter and concentrate. Use 2M NH by prepping the plate 3 MeOH / CH 2 Cl 2 Solution The crude product was purified twice to give 37 mgs of the title product. MS 384.3 (M+H); LCMS condition A, retention time 1.31 min. 1H NMR (400MHz, CDCl 3 )δppm 1.83(t, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com