Inorganic phase-change material and preparation method thereof
An inorganic phase change material and quality technology, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of latent heat of phase change and cycle performance that have not been studied, and achieve small performance differences, simple and easy operation, and repeatability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Put 100g of sodium sulfate decahydrate and 100g of disodium hydrogen phosphate dodecahydrate into 18g of water and mix between 40~45 o C. The hydrated salt is completely melted in the water bath. Add 10g of sodium pyrophosphate decahydrate and stir until a clear solution is formed. Add 0.02g of sodium polyacrylate and stir evenly, then add 10g of super absorbent resin, stir to make the super absorbent resin completely The solution is absorbed to obtain the phase change material of the present invention.
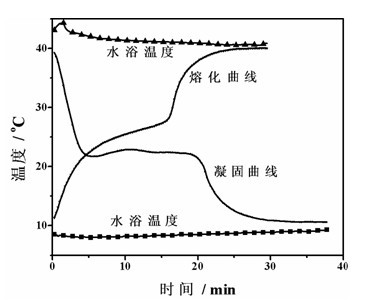
[0040] Take 20g of the phase change material obtained and put it in a test tube with a diameter of 25mm, and place the test tube in 8 o In C water, every 15 seconds, use a temperature sensor to record the temperature of the phase change material. Use temperature as the vertical axis and time as the horizontal axis to draw the solidification curve of the phase change material, such as figure 1 Shown. After measuring the solidification curve, place the test tube at 40 o In w...
Embodiment 2
[0042] Put 130g sodium sulfate decahydrate and 70g disodium hydrogen phosphate dodecahydrate into 40g water, and mix in 40~45 o In the C water bath, the hydrated salt is completely melted, 6g sodium pyrophosphate decahydrate is added and stirred until a clear solution is formed, 12g super absorbent resin is added, and the super absorbent resin is stirred to completely absorb the solution to obtain the phase change material of the present invention.
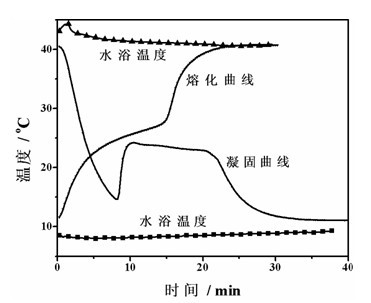
[0043] The solidification-melting curve of the phase change material of this embodiment was measured by the same method as in Example 1, as figure 2 Shown. It can be seen from the figure that the phase change material of this embodiment is o It solidifies at C, the supercooling is larger, at 22~25 o C melts.
Embodiment 3
[0045] Put 120g sodium sulfate decahydrate and 80g disodium hydrogen phosphate dodecahydrate into 32.9g water, and mix in 40~45 o Melt the hydrated salt in the C water bath, add 12g of sodium pyrophosphate decahydrate and stir until a clear solution is formed, add 6g of boric acid, the pH is reduced from 9.03 to 7.31, add 10g of super absorbent resin, stir to make the super absorbent resin completely absorb the solution. That is, the phase change material of the present invention is obtained.
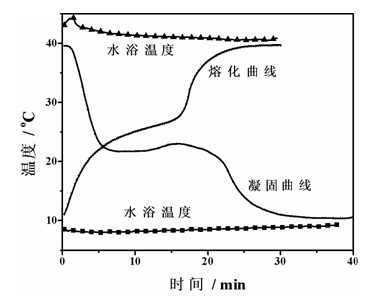
[0046] The solidification-melting curve of the phase change material of this embodiment was measured by the same method as in Example 1, as image 3 Shown. It can be seen from the figure that the phase change material of this embodiment is o Solidification at C, no overcooling, at 22~25 o C melts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition enthalpy | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com