A kind of inorganic phase change material and preparation method thereof
An inorganic phase change material and quality technology, applied in heat exchange materials, chemical instruments and methods, etc., can solve the problems of phase change latent heat and cycle performance that have not been studied, and achieve small performance differences, simple methods and easy operation, repeatability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 100g of sodium sulfate decahydrate and 100g of disodium hydrogen phosphate dodecahydrate into 18g of water, and o C water bath to completely melt the hydrated salt, add 10g of sodium pyrophosphate decahydrate and stir until a clear solution is formed, then add 0.02g of sodium polyacrylate and stir evenly, then add 10g of superabsorbent resin, and stir to make the superabsorbent resin completely The solution is absorbed to obtain the phase change material of the present invention.
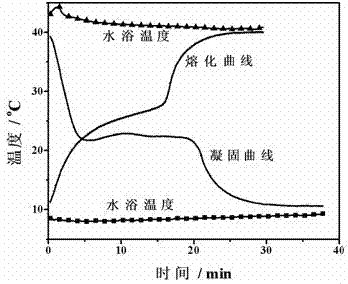
[0038] Get 20g of the gained phase-change material and put it into a test tube with a diameter of 25mm, place the test tube at 8 o In C water, every 15 seconds, record the temperature of the phase-change material with a temperature sensor, take the temperature as the vertical axis, and the time as the horizontal axis, and draw the solidification curve of the phase-change material, as figure 1 shown. After measuring the coagulation curve, place the test tube at 40 o In water C, use the ...
Embodiment 2
[0040] Put 130g of sodium sulfate decahydrate and 70g of disodium hydrogen phosphate dodecahydrate into 40g of water, and o C water bath to completely melt the hydrated salt, add 6g of sodium pyrophosphate decahydrate and stir until a clear solution is formed, add 12g of superabsorbent resin, stir to make the superabsorbent resin completely absorb the solution, and obtain the phase change material of the present invention.
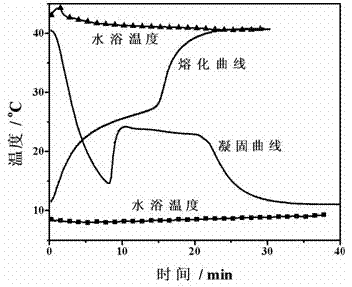
[0041] Adopt the same method as embodiment 1 to measure the solidification-melting curve of the phase change material of this embodiment, such as figure 2 shown. It can be seen from the figure that the phase change material in this embodiment is between 22 and 23 o It solidifies at C, and the supercooling is relatively large, at 22-25 o C melts.
Embodiment 3
[0043] Put 120g of sodium sulfate decahydrate and 80g of disodium hydrogen phosphate dodecahydrate into 32.9g of water, and o Melt the hydrated salt in a C water bath, add 12g of sodium pyrophosphate decahydrate and stir until a clear solution is formed, add 6g of boric acid, the pH drops from 9.03 to 7.31, add 10g of superabsorbent resin, stir to make the superabsorbent resin completely absorb the solution, That is, the phase change material of the present invention is obtained.
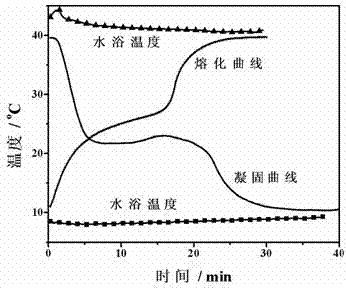
[0044] Adopt the same method as embodiment 1 to measure the solidification-melting curve of the phase change material of this embodiment, such as image 3 shown. It can be seen from the figure that the phase change material in this embodiment is between 22 and 23 o C solidification, no supercooling, at 22 ~ 25 o C melts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition enthalpy | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
| enthalpy of fusion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com