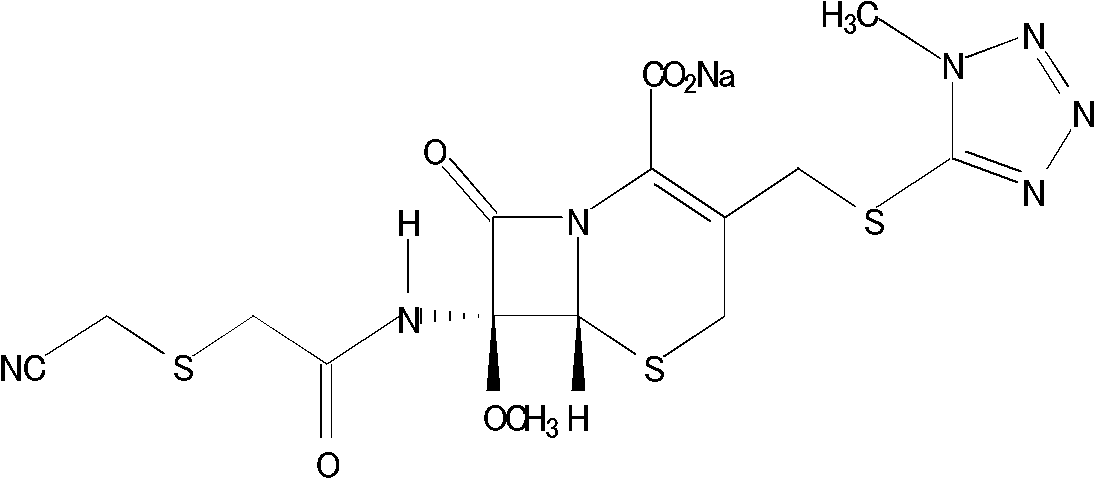
Cefmetazole sodium crystal compound, preparation method thereof and sterile powder for injection containing cefmetazole sodium crystal compound
A technology of cefmetazole sodium and crystal compound, applied in the field of chemical pharmacy, can solve the problems of poor uniformity of mixed powder, high cost, poor fluidity, etc., and achieve the effects of improving particle size distribution, adjusting crystal particle size, and shortening crystallization time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] [Example 1] Preparation of Cefmetazole Sodium Crystals
[0045] 1) Prepare the crude product solution: dissolve 20 kg of the crude product of cefmetazole sodium in 10 L of water, control the temperature at about 20° C., add 0.01% of the weight of the crude product of cefmetazole sodium for decolorization, and filter to obtain 2.0 kg / L of cefmetazole sodium aqueous solution;
[0046] 2) Crystal nucleation process: control the temperature of the cefmetazole sodium aqueous solution at about 20°C, add 50L of absolute ethanol to the aqueous solution at a rate of 10ml / min under stirring at a stirring speed of 130r / min, and turbidity appears , to obtain a cloudy solution;
[0047] 3) Crystal growth process: place the turbid solution obtained in step 2) at a frequency of 3.5kHz and an intensity of 0.6W·cm -2 Under the ultrasonic field, the temperature of the solution is controlled at about 20°C, and a mixed solution of 70L of acetone and tetrahydrofuran is added dropwise at a...
Embodiment 2-10
[0051]
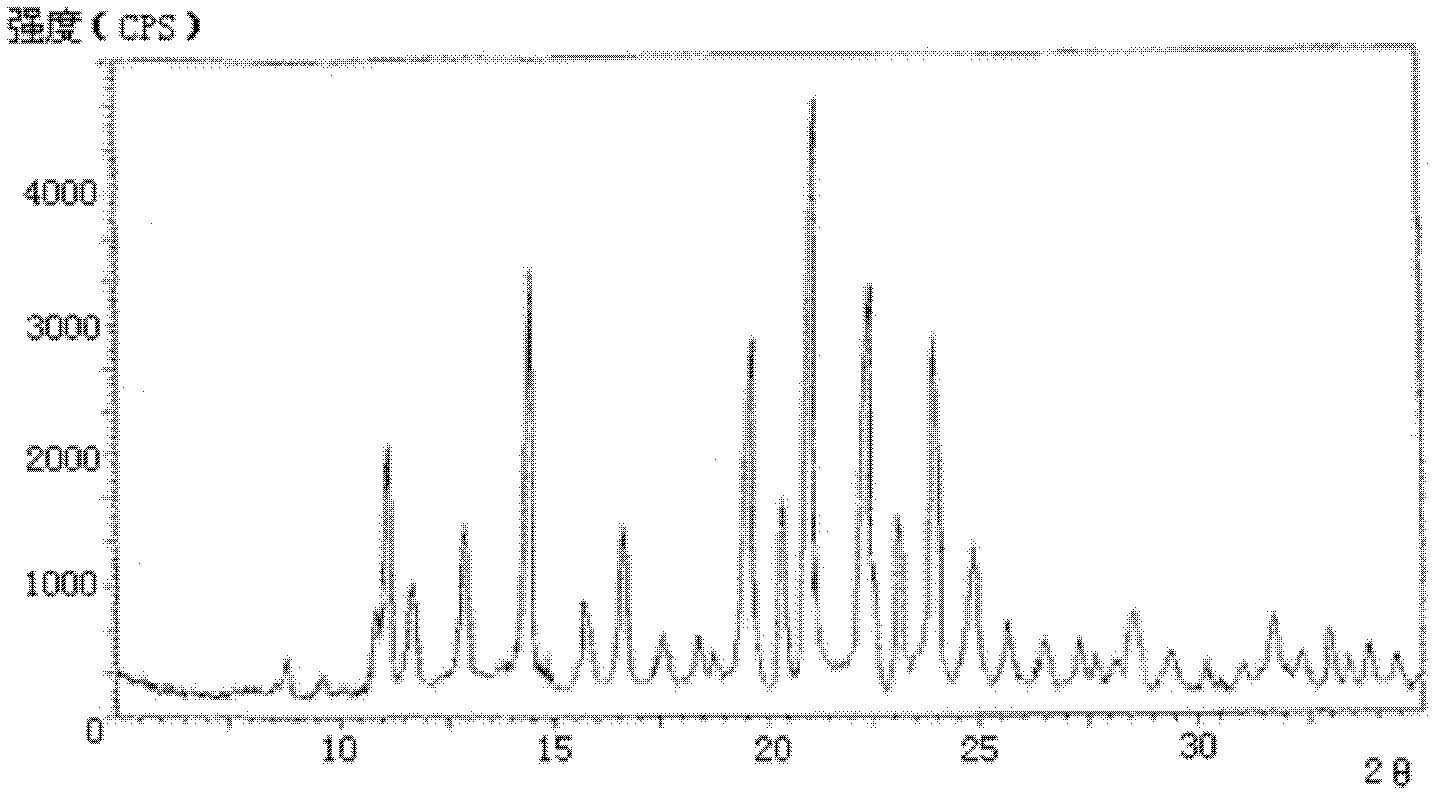
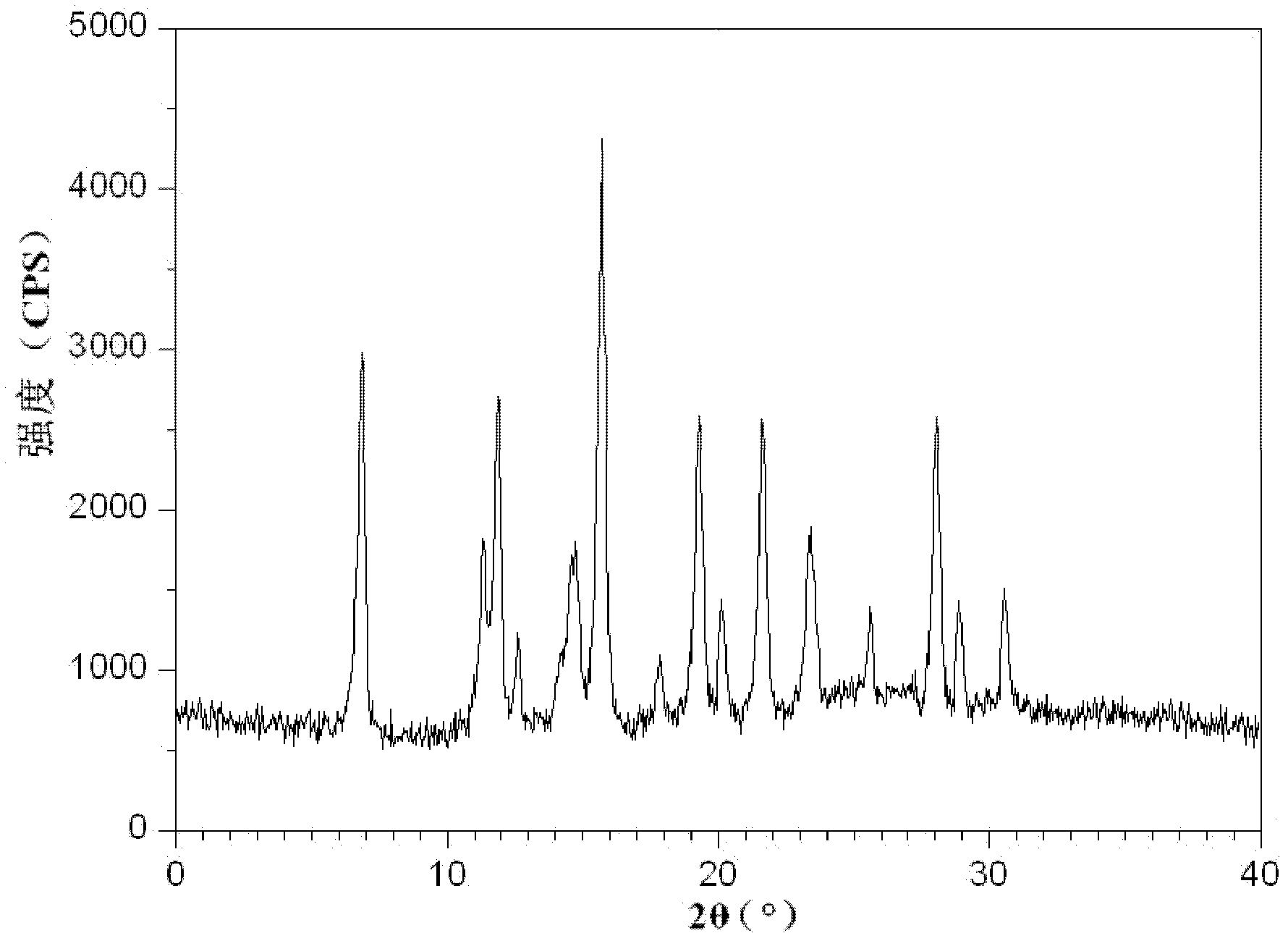
[0052] The X-ray powder diffraction pattern obtained by measuring the cefmetazole sodium crystals prepared in Examples 2-10 using Cu-K α rays is consistent with that of Example 1.
preparation Embodiment 1
[0053] [Preparation Example 1] Cefmetazole Sodium Composition Sterile Powder for Injection
[0054] Specification: 1.0g (in C 15 h 17 N 7 o 5 S 3 count)
[0055] prescription:
[0056] Cefmetazole sodium crystal 1000g prepared by embodiment 1 (in C 15 h 17 N 7 o 5 S 3 count)
[0057] A total of 1000 bottles were made
[0058] Preparation:
[0059] Get the cefmetazole sodium crystal 1000g prepared by embodiment 1 (in C 15 h 17 N 7 o 5 S 3 Calculated), under aseptic conditions, it is divided into 1000 bottles of antibiotic glass bottles, and the amount of each bottle is 1.0g (in C 15 h 17 N 7 o 5 S 3 meter), stoppering, capping, light inspection, inspection qualified, labeling, packaging to obtain the sterile powder of cefmetazole sodium composition for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com