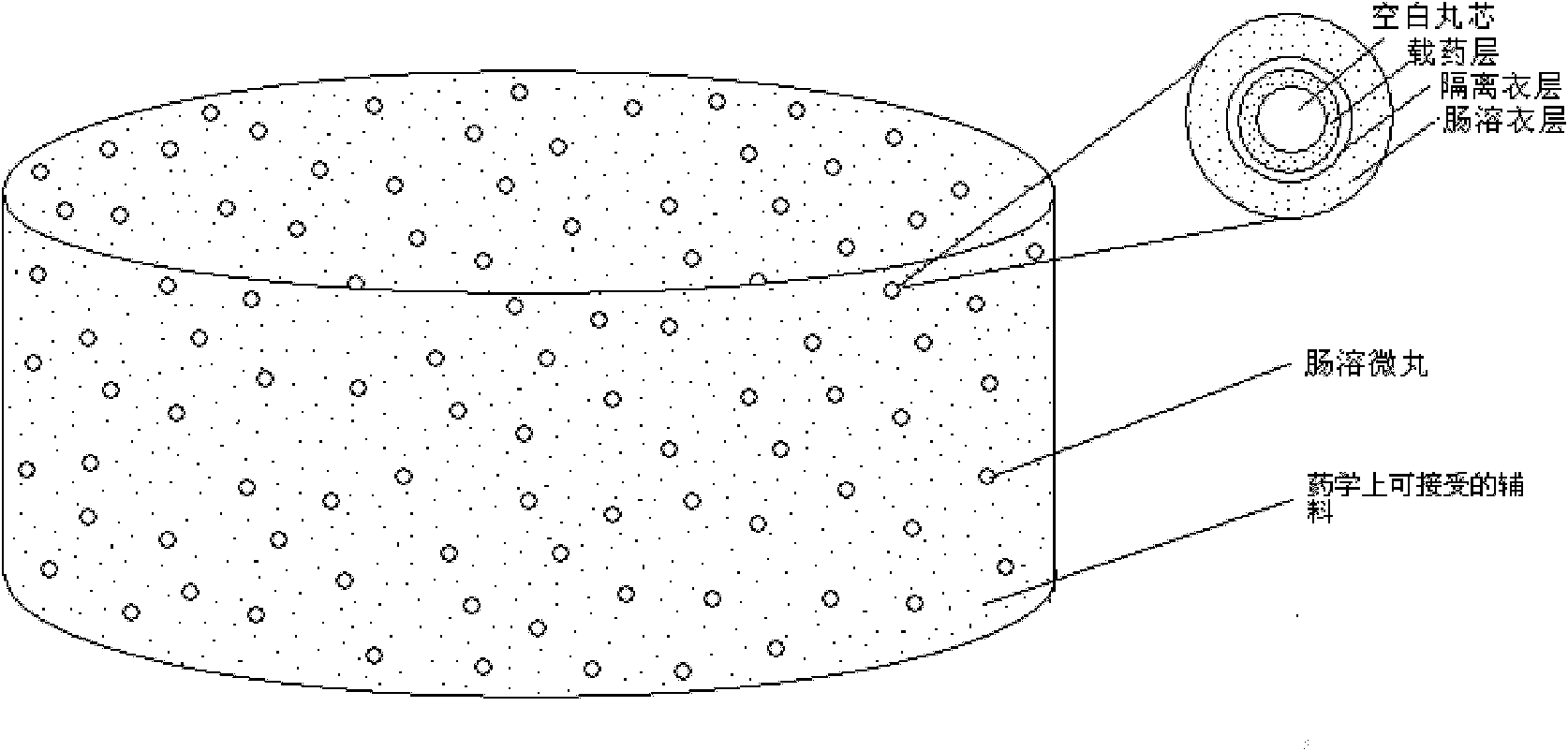
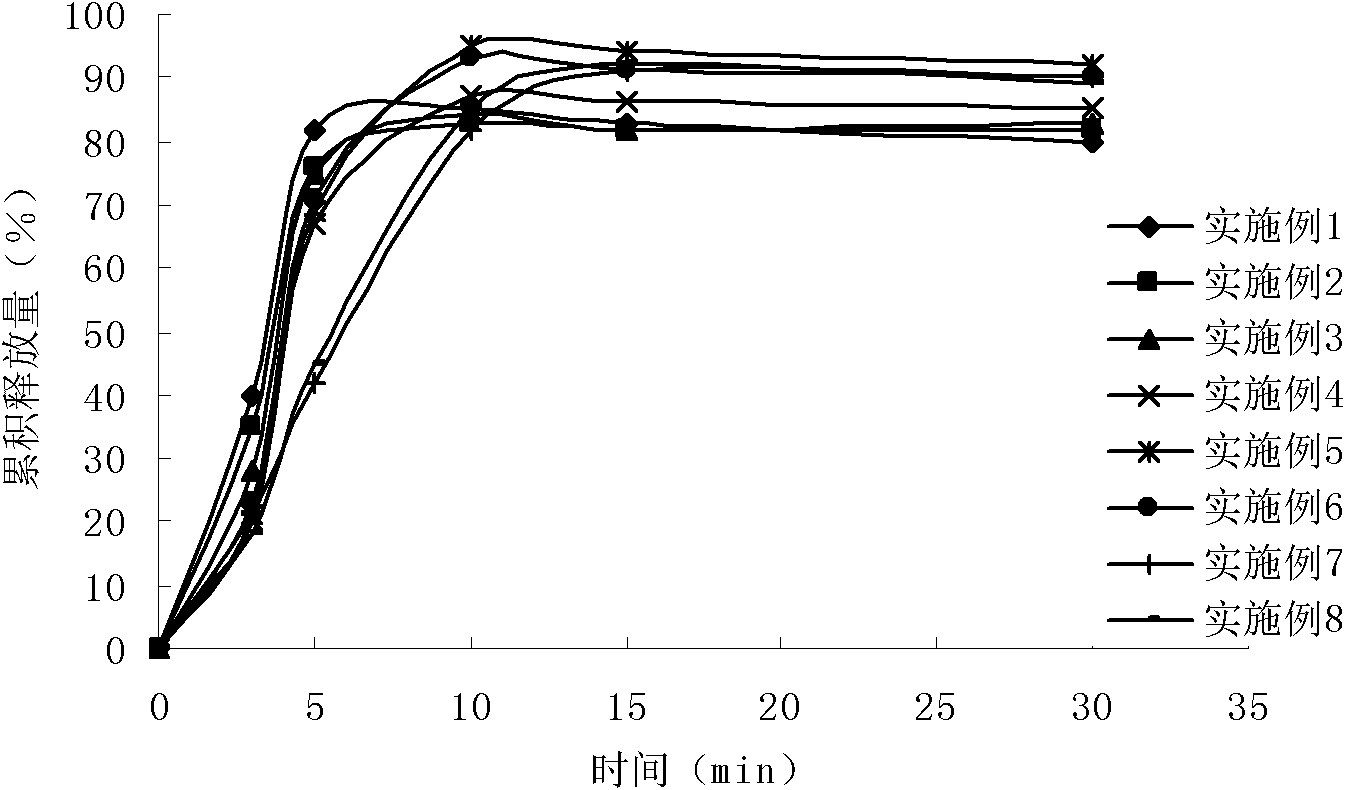
Rabeprazole sodium enterosoluble micro-particles and preparation method thereof
A technology of rabeprazole sodium and enteric-coated microparticles is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. , Unable to judge the pros and cons of acid resistance, poor acid resistance of tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0084] The present invention will be further clarified below in conjunction with embodiments, and the purpose, features and advantages of the present invention will become apparent to those skilled in the art through the following description. The examples listed below can be used to help understand the present invention, and should not be considered as limiting the scope of the present invention.
[0085] Unless otherwise indicated, all percentages are percentages by weight.
[0086] 1. Prescription
[0087] Table 1 prescription composition
[0088]
[0089]
[0090]
[0091] 2. Preparation method
[0092] 1. Preparation of drug-loaded particles
[0093] ① Preparation of the drug-loading solution: Weigh the components of the drug-loading layer composition according to the prescription in Table 1 above, disperse and dissolve in the 80% ethanol-sodium hydroxide solution, stir well, and set aside.
[0094] ②Drug loading: Weigh blank pellets according to the prescription in Table 1 above, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com