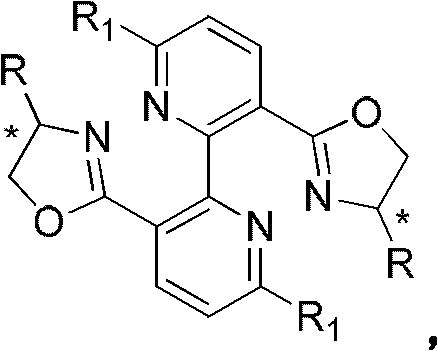
Axially unstable bipyridine-bisoxazoline chiral ligands and their preparation and application
A bisoxazoline and axially unstable technology, which is applied to axially unstable bipyridine-bisoxazoline chiral ligands and the fields of preparation and application thereof, can solve the waste of axially chiral ligands, and only has a central hand. Sexual and chiral ligands are expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
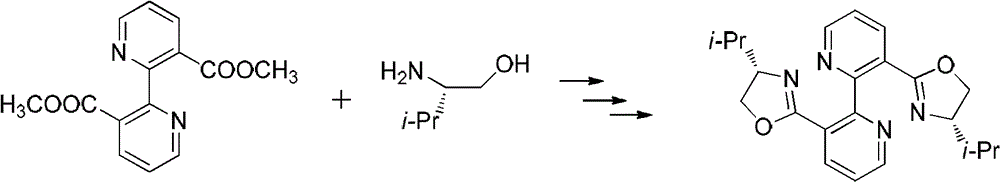
[0025] Embodiment 1: the synthesis of bipyridyl-isopropyl oxazoline
[0026]
[0027] Under the condition of nitrogen protection, 2.72 g (0.01 mol) of methyl bipyridylcarboxylate and 4.12 g (0.04 mol) of s-isopropylamino alcohol were added to the system, heated to 120°C and stirred for 20 hours, then cooled to room temperature, Then add 30ml of dichloromethane, 5ml of thionyl chloride and reflux for 6 hours, then evaporate the solvent under reduced pressure, dissolve the obtained viscous substance in 15ml of methanol, add dropwise to 15ml of methanol solution of 2.6g of sodium hydroxide, and reflux for 6 hours. After one hour, the solvent was evaporated under reduced pressure, 50 ml of dichloromethane was added to dissolve, washed with saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and passed through a column with petroleum ether / ethyl acetate 2:1 to obtain 1.93 g of a white solid , yield 51%.
[0028] 1 H ...
Embodiment 2
[0029] Embodiment 2: the synthesis of bipyridyl-phenyloxazoline
[0030]
[0031]Under the condition of nitrogen protection, add 2.72g (0.01mol) of methyl bipyridylcarboxylate and 5.49g (0.04mol) of s-phenylamino alcohol to the system, heat to 120°C and stir for 20 hours, then cool to room temperature, and then Add 30ml of dichloromethane, 5ml of thionyl chloride and reflux for 6 hours, then evaporate the solvent under reduced pressure, dissolve the obtained viscous substance in 15ml of methanol, add dropwise to 2.6g of sodium hydroxide in 15ml of methanol solution, and reflux for 6 hours After that, the solvent was evaporated under reduced pressure, 50 ml of dichloromethane was added to dissolve, washed with saturated sodium carbonate solution, saturated sodium chloride solution, dried over anhydrous sodium sulfate, and petroleum ether / ethyl acetate 2:1 was passed through the column to obtain 2.50 g of white solid. Yield 56%.
[0032] 1 H NMR (400MHz, CDCl 3 ): δ8.75(s,...
Embodiment 3
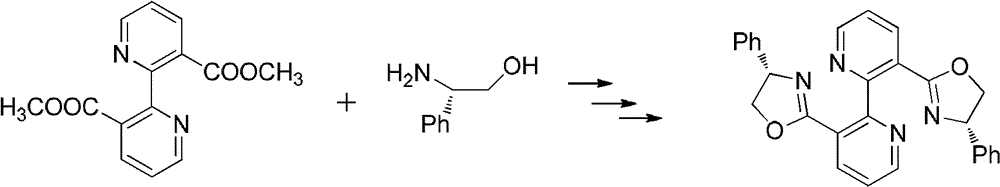
[0033] Embodiment 3: the synthesis of bipyridine-benzyl oxazoline
[0034]
[0035] Under the condition of nitrogen protection, 2.72g (0.01mol) of methyl bipyridylcarboxylate and 6.05g (0.04mol) of s-benzylamino alcohol were added to the system, heated to 120°C and stirred for 20 hours, cooled to room temperature, and then Add 30ml of dichloromethane, 5ml of thionyl chloride and reflux for 6 hours, then evaporate the solvent under reduced pressure, dissolve the obtained viscous substance in 15ml of methanol, add dropwise to 2.6g of sodium hydroxide in 15ml of methanol solution, and reflux for 6 hours Afterwards, the solvent was evaporated under reduced pressure, and 50 ml of dichloromethane was added to dissolve, washed with saturated sodium carbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and passed through a column with petroleum ether / ethyl acetate 2:1 to obtain 2.94 g of a white solid. Yield 62%.
[0036] 1 H NMR (400MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com