GPC3 (glypican-3) monoclonal antibody hybridoma strain 8G6, and preparation method and application thereof
A hybridoma cell line and monoclonal antibody technology, applied in the field of cell engineering, can solve the problems of limited protein application and high price, and achieve the effects of easy survival, reduced production cost, and sensitive and easy response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of GPC3 recombinant protein
[0025] 1. Construction of GPC3 / PET-11b recombinant vector
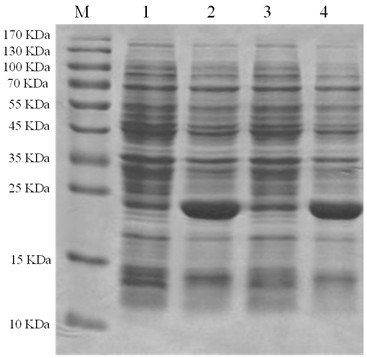
[0026] According to the human GPC3 mRNA (NM_001164619) sequence provided in GenBank, the N-terminal signal peptide was removed, and a pair of primers were designed for bases 72-369 of the sequence. The upstream primer: 5' TAATGAATTCATGCAGCCCCCGCCGCC 3' (SEQ ID NO: 1) downstream primer: 5' AGCGGTCGACTCACTTGGCATGGCGAACAACAA 3' (SEQ ID NO: 2). Introduced at the 5' ends of the two primers Eco RI and Sal Ⅰ Restriction site, using the conventional PCR method to use the cDNA reverse-transcribed from the total RNA of Huh7 (purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences) as a template to transfer the GPC3 N-terminal gene, and agarose gel electrophoresis showed that the GPC3 N-terminal was successfully obtained gene, the fragment length was as expected ( figure 1 ).
[0027] The prokaryotic expression vector PET-11b (purchased from Mer...
Embodiment 2
[0033] Example 2 Preparation of mouse anti-human GPC3 monoclonal antibody
[0034] 1. Establishment of Hybridoma Cell Lines
[0035] (1) Immunization of mice
[0036] The purified GPC3 recombinant protein was mixed with 250 μL of Bentonite adjuvant, and BALB / c mice were subcutaneously injected into BALB / c mice at 100 μg / 500 μL, and BALB / c mice were injected subcutaneously at 100 μg / 500 μL at intervals of three weeks. c mice, starting from the third immunization, the mouse blood was collected from the tail vein in the second week after each immunization, and prepared for fusion after the serum titer was detected by indirect ELISA method to reach 1:50,000 or more. Three days before the fusion, the tail vein was injected Booster immunization once, antigen dose 100 μg.
[0037] (2) Determination of immune serum titer
[0038] The titer of immune serum was determined by indirect ELISA. Dissolve 30 μg of GPC3 recombinant protein in 10 mL of 0.05 M pH9.6 carbonate buffer, coat ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com