Method for online detection of concentration of electrolyte of vanadium battery
A technology of electrolyte concentration and vanadium battery, which is applied in the direction of measuring device, color/spectral characteristic measurement, material analysis by optical means, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Take random samples, dilute 100 times, test the absorbance of the sample, and draw its ultraviolet-visible spectrum curve, compare the sample spectrum curve with the standard spectrum curve of monovalent state and mixed state vanadium ion, according to the two The shape of the curve and the position of the absorption peak determine that the sample is a trivalent vanadium ion solution, and the ultraviolet absorbance A at 401nm is taken u 401.0nm =0.409, into the curve equation of the absorbance and concentration of the trivalent vanadium ion solution deduced by the present invention, the concentration C of the trivalent vanadium ion can be obtained (III) =0.08563A u 401.0nm +5.21×10 -5 =0.03507(mol / L), or take the UV absorbance A at 606.5nm u 606.5nm =0.25199, substituting in the curve equation of the absorbance and concentration of the trivalent vanadium ion solution deduced by the present invention, obtains the concentration C of trivalent vanadium ion (III) =0.1...
Embodiment 2
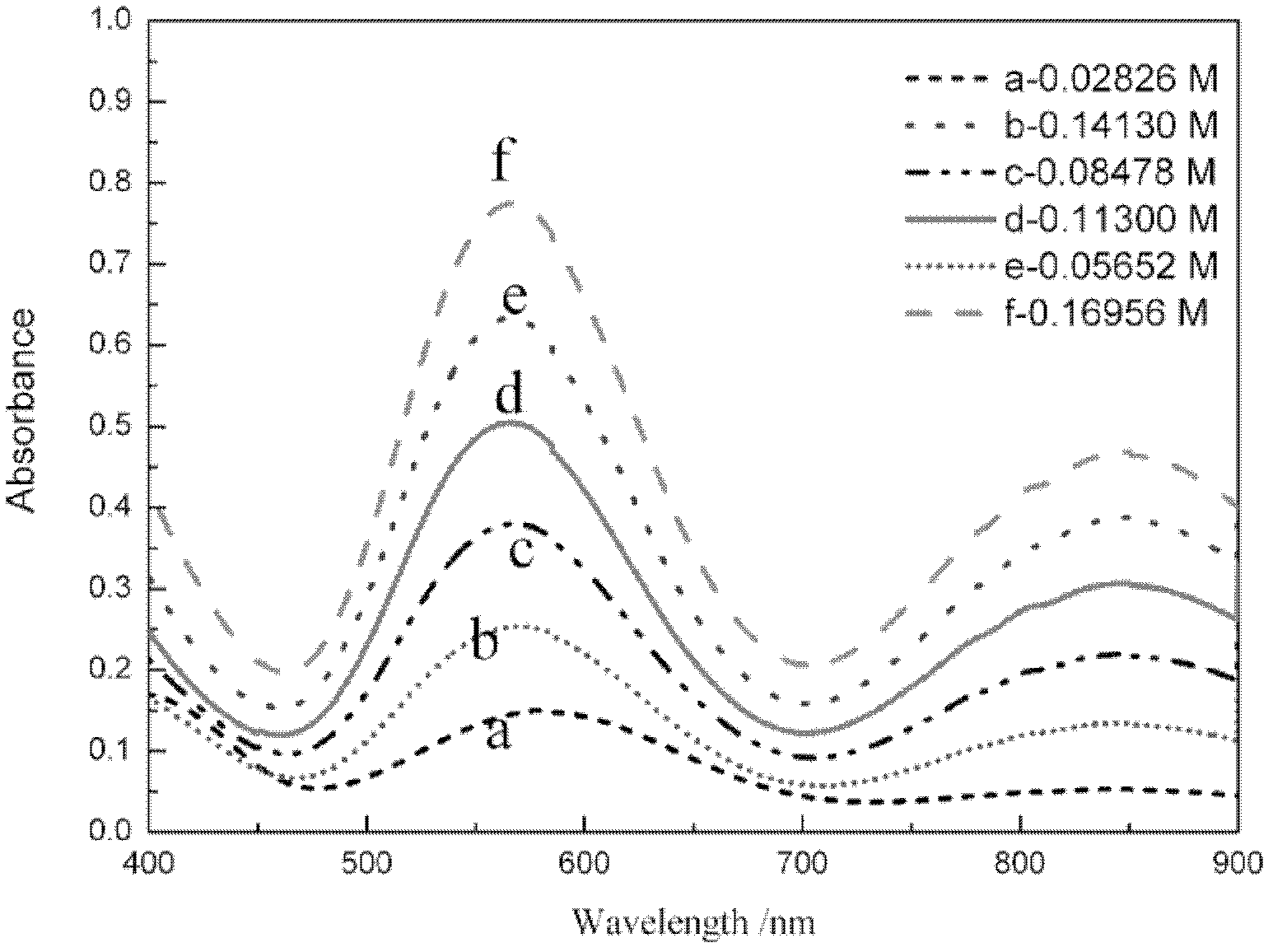
[0127] Take random samples, dilute 100 times, test the absorbance of the sample, and draw its ultraviolet-visible spectrum curve, compare the sample spectrum curve with the standard spectrum curve of monovalent state and mixed state vanadium ion, according to the two The shape of the curve and the position of the absorption peak determine that the sample is a divalent vanadium ion solution, and the ultraviolet absorbance A at 572.5nm is taken u 572.5nm =0.5025, into the curve equation of the absorbance and concentration of the divalent vanadium ion solution deduced by the present invention, the concentration C of the divalent vanadium ion can be obtained (II) =0.2267A u 572.5nm -2.37×10 -3 =0.1115 (mol / L), the obtained experimental result is compared with the concentration 0.113mol / L measured by titration, and the error is within 5%, see Table 1.
Embodiment 3
[0129] Take random samples, dilute 100 times, test the absorbance of the sample, and draw its ultraviolet-visible spectrum curve, compare the sample spectrum curve with the standard spectrum curve of monovalent state and mixed state vanadium ion, according to the two The shape of the curve and the position of the absorption peak determine that the sample is a tetravalent vanadium ion solution, and the ultraviolet absorbance A at 770nm is taken u 770nm =0.73211, substituting in the curve equation of the absorbance and concentration of the tetravalent vanadium ion solution deduced by the present invention, can obtain the concentration C of tetravalent vanadium ion (IV) =0.0546A u 770nm -1.38×10 -4 =0.0389 (mol / L), the obtained experimental result is compared with the concentration 0.04mol / L measured by titration, and the error is within 5%, see Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com