Cyclic polymer and preparation method thereof
A technology for cyclic polymers and compounds, applied in the field of cyclic polymers and their preparation, to achieve the effects of enhanced fluorescence and grating performance, strong fluorescence emission, and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Such as figure 1 Preparation shown cycle -PEPNA, cycle The synthesis of c-PEPNA is divided into 3 main steps: Synthesis of azide / alkynyl main chain phenylazonaphthalene monomer EPNA through reaction steps such as diazo and coupling; "Click" step-by-step polymerization of EPNA by solid-phase thermocatalysis method to obtain α-azido-ω-alkynyl linear -PEPNA; In very dilute solution, by the CuAAC method for linear -PEPNA has carried out ring closure reaction, obtains cycle -PEPNA. The number average molecular weight and molecular weight distribution of the polymers are shown in Table 1.
[0037] Specifically include the following steps:
[0038] 1. Synthesis of compound (1)
[0039] References (Xue, X. Q., Zhu, J., Zhu, X. L., et al., polymer 2009, 50, 4512) Synthesis. The crude product was recrystallized from ethanol to obtain an orange-red solid (12.6 g, yield, 92.6%).
[0040] 2. Synthesis of compound (2)
[0041] Filled with CaCl in 250mL 2 Add co...
Embodiment 2
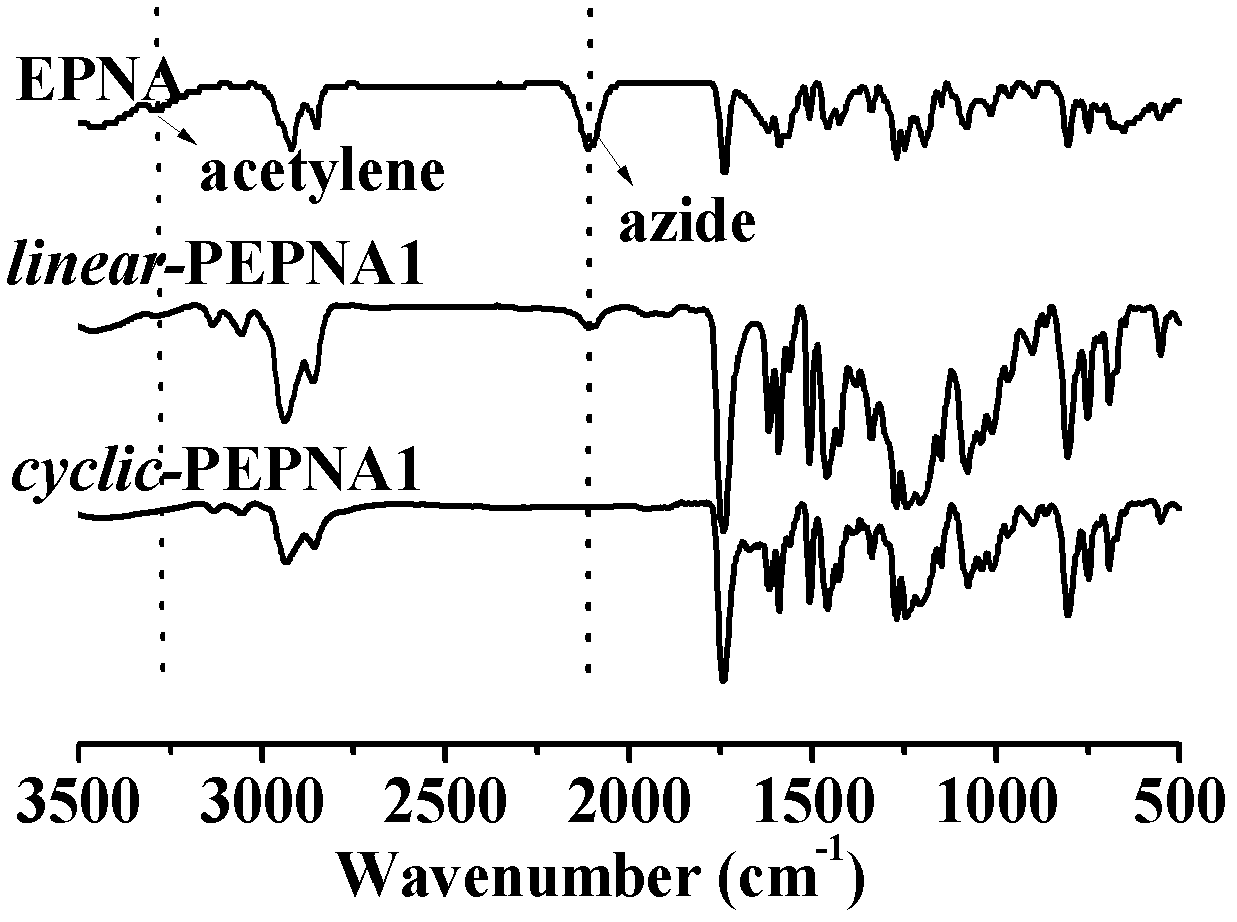
[0053] Embodiment two: to embodiment one linear -PEPNA and cycle -PEPNA for structural characterization. linear -PEPNA and cycle -The efflux curve of the GPC of PEPNA (see figure 2 ), you can see two cycle - The retention times of the elution peaks of -PVBCZ were longer than those of the corresponding linear -Long duration of PEPNA. This is due to the smaller hydrodynamic volume of cyclic polymers than their linear counterparts of the same molecular weight (see references: Rique-Lurbet L., Schappacher M, and Deffieux A.. Macromolecules 1994, 27, 6318-6324). From the FT-IR spectrum (see image 3 ), it can be seen that the linear polymer is at 2090cm -1 The azide peak at and 3300cm -1 The alkynyl peaks at have disappeared after the cyclization reaction, proving that cycle - There are no linear polymers with terminal groups in PEPNA. Figure 4 yes linear -PEPNA and Cyclic - H NMR spectrum of PEPNA. Comparing the two, one can see linear - All alkynyl hydroge...
Embodiment 3
[0054] Embodiment three: linear -PEPNA and Cyclic -PEPNA thermal performance test
[0055] tested using differential scanning calorimetry linear -PEPNA and cycle -PEPNA T g ,As shown in Table 1: cycle -PEPNAs T g have the same molecular weight than their corresponding linear -PEPNAs are higher. As the molecular weight decreases, the gap between the ring and the line T g The difference increases. This is due to the lack of swinging chain ends in the molecular chain of the cyclic polymer, so the degree of freedom of the cyclic polymer to transition from the solid state to the relaxed state is less, resulting in the T g Higher than the linear polymer of the same molecular weight; as the molecular weight of the cyclic polymer decreases, that is, the size of the ring decreases, the rigidity of the ring increases, and the Δ between the cyclic polymer and the linear polymer T g increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com