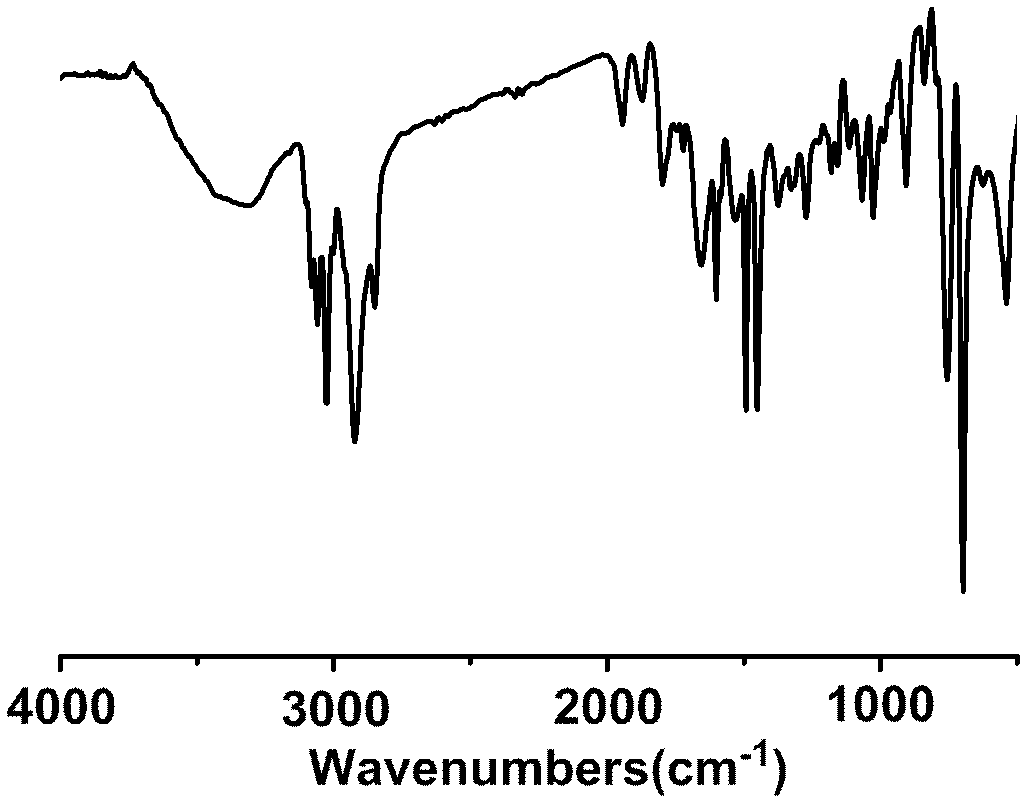
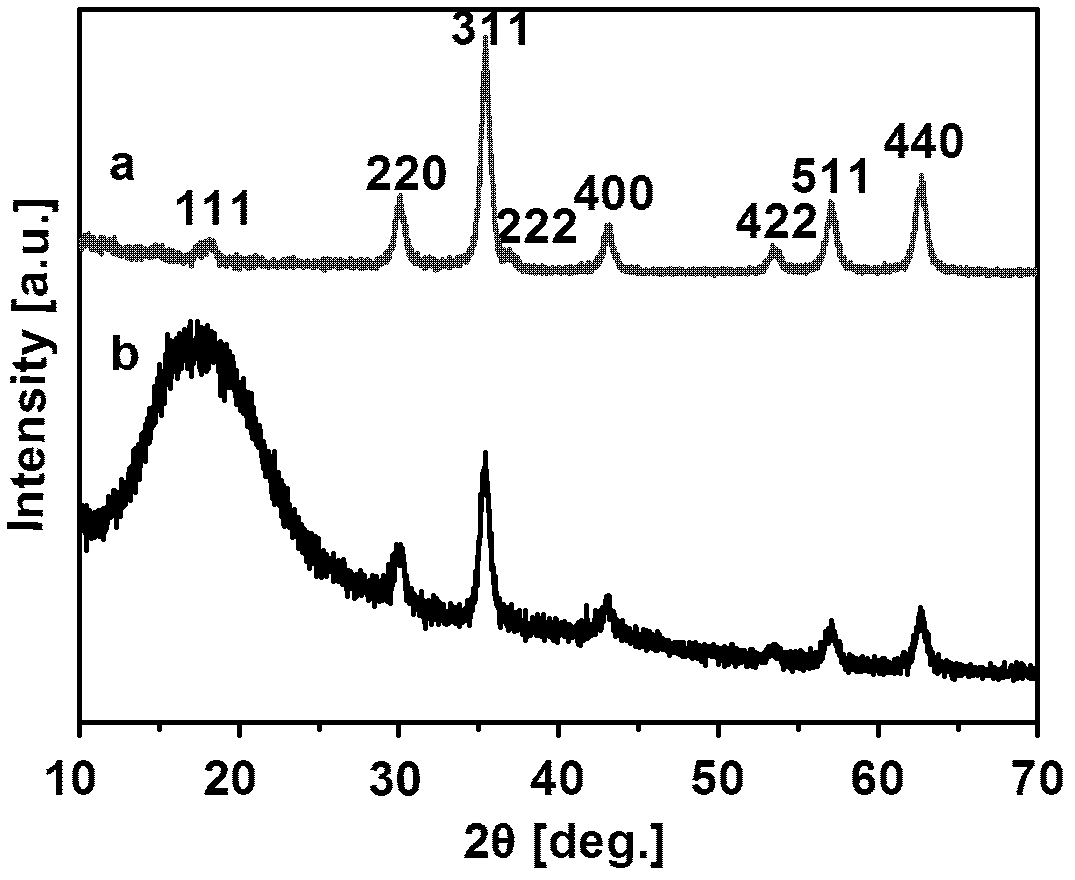
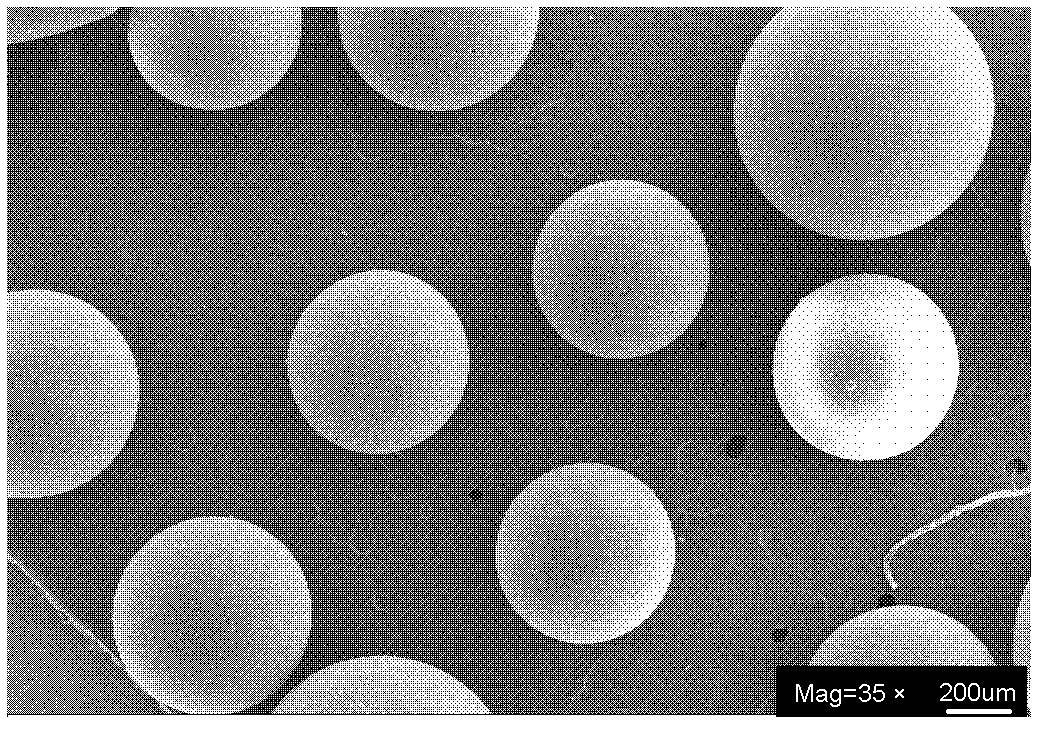
Preparation method for chiral magnetic microspheres containing substituted acetylene polymer
A technology of acetylene polymers and magnetic microspheres, applied in organic chemistry methods, chemical instruments and methods, separation of optically active compounds, etc., can solve problems such as limited applicability, cumbersome operation, and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1: The first step is to prepare magnetic nanoparticles with double bonds. The details are as follows: Add 5.838g of ferric chloride hexahydrate and 3.003g of ferrous sulfate heptahydrate into a 250ml three-necked bottle, and dissolve with 100ml of deionized water. At 85° C., under nitrogen protection, under vigorous mechanical stirring, 25 wt % ammonia solution was added to adjust the pH of the solution to 10-11, and a black precipitate was formed immediately. After reacting for half an hour, the black precipitate obtained was repeatedly washed with ethanol to obtain ferric oxide nanoparticles. The nanoparticles are modified with a silane coupling agent KH-570 to obtain ferric oxide nanoparticles with double bonds on the surface. Disperse 1.000g of iron ferric oxide nanoparticles in a mixed solution of 380ml of ethanol and 20ml of water adjusted by glacial acetic acid at a pH of 4-5, mix well by ultrasonic, add 8ml of silane coupling agent KH-570, and react at ...
example 2
[0044] Example 2: The first step is to prepare magnetic nanoparticles with double bonds, and the specific steps are the same as in Example 1.
[0045] The second step synthesizes the macromonomer with double bond, at first 0.005g monomer M1 with double bond and 0.115g chiral alkyne monomer M2b are dissolved in 0.5ml chloroform, 0.002g rhodium metal catalyst is dissolved in In 0.5ml of chloroform, vacuumize and inflate nitrogen three times respectively, and then transfer the solution of rhodium metal catalyst to the solution of alkyne monomer under the protection of nitrogen, and react at 30°C for 2 hours to obtain polyalkyne helical macromonomer .
[0046] The third step is to prepare chiral magnetic microspheres containing substituted acetylene polymers. Add 1ml of polyacetylene helical macromer, 0.488g of styrene, 0.012g of divinylbenzene, 0.008g of azobisisobutyronitrile, and 0.015g of ferric oxide nanoparticles with double bonds into a 100ml three-necked flask, and ultras...
example 3
[0048] Example 3: The first step is to prepare magnetic nanoparticles with double bonds, and the specific steps are the same as in Example 1.
[0049] The second step synthesizes the macromonomer with double bond, at first 0.005g monomer M1 and 0.124g chiral alkyne monomer M2c with double bond are dissolved in 0.5ml chloroform, 0.002g rhodium metal catalyst is dissolved in In 0.5ml of chloroform, vacuumize and inflate nitrogen three times respectively, and then transfer the solution of rhodium metal catalyst to the solution of alkyne monomer under the protection of nitrogen, and react at 30°C for 2 hours to obtain polyalkyne helical macromonomer .
[0050] The third step is to prepare chiral magnetic microspheres containing substituted acetylene polymers. Add 1ml of polyacetylene helical macromer, 0.488g of styrene, 0.012g of divinylbenzene, 0.008g of azobisisobutyronitrile, and 0.015g of ferric oxide nanoparticles with double bonds into a 100ml three-necked flask, and ultras...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com