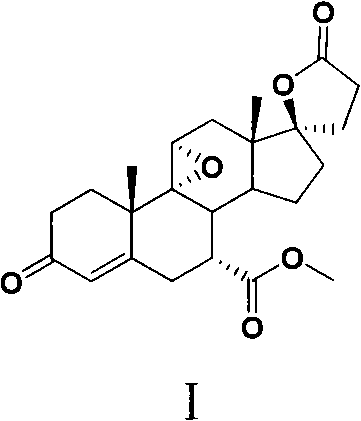
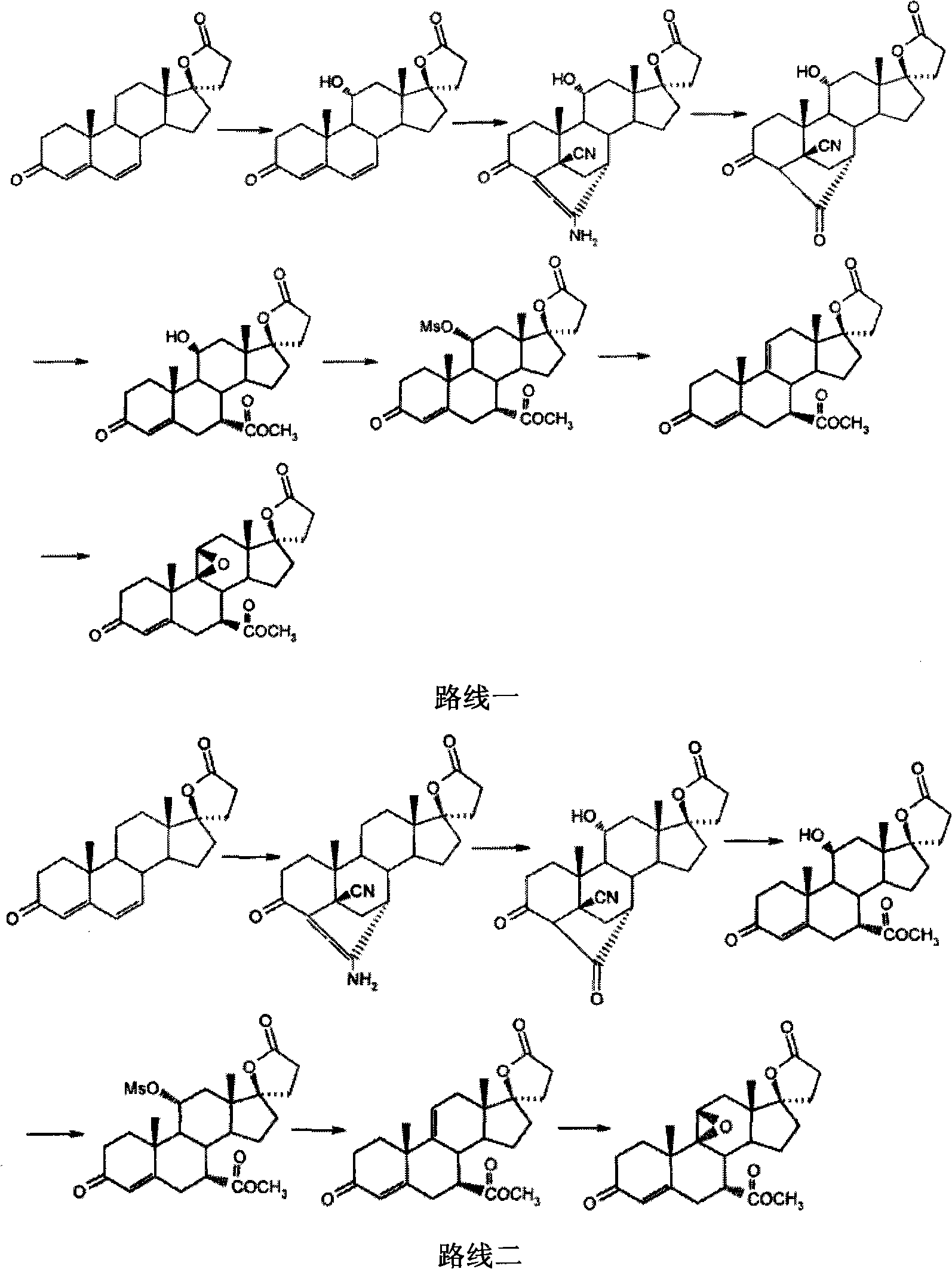
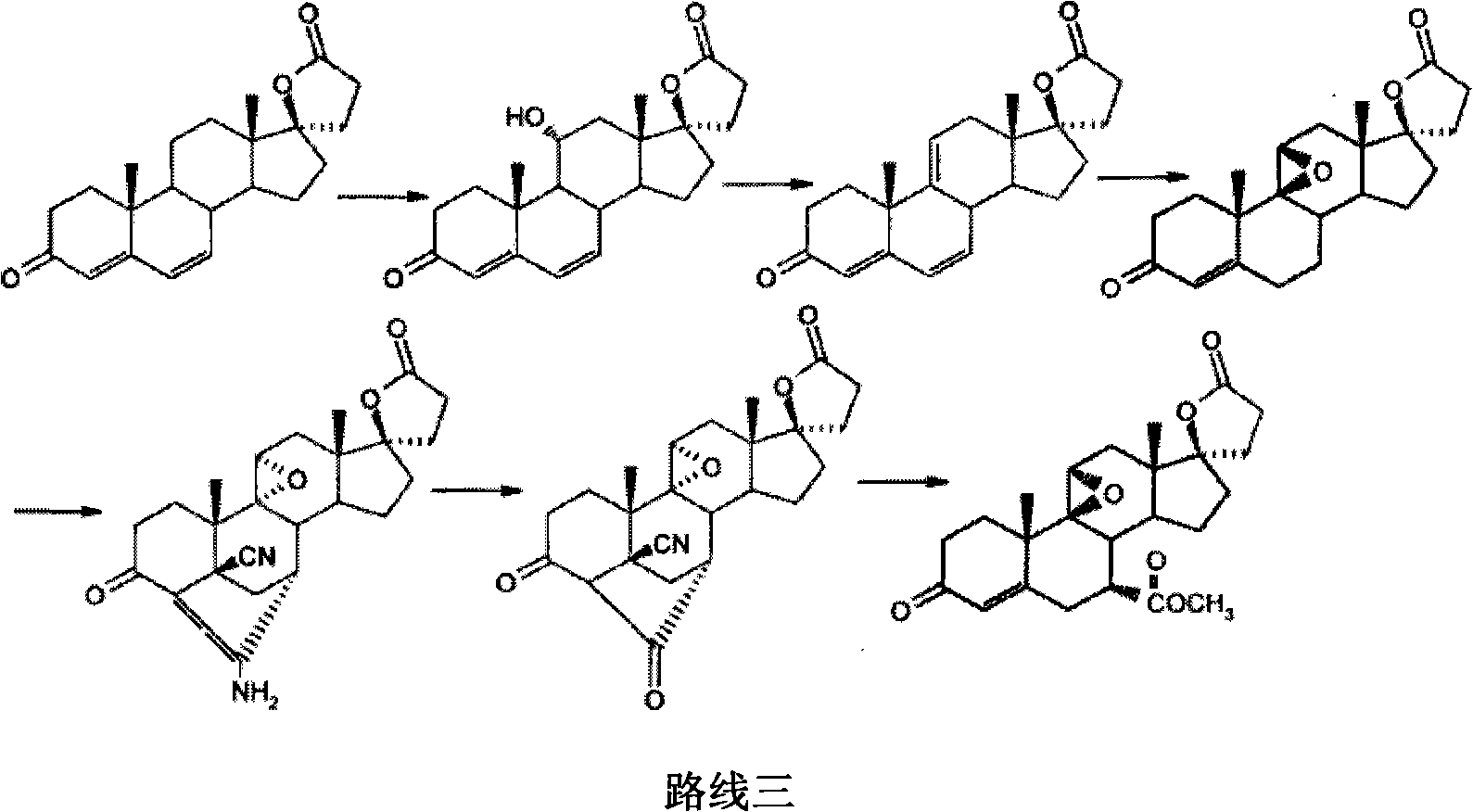
New method for preparing eplerenone
A technology of eplerenone and androstenedione, which is applied in the field of preparation of new steroidal aldosterone receptor antagonist eplerenone, achieves the effect of cheap source, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 4,9(11)-Androstadiene-3,17-dione (III)
[0039] Under nitrogen protection, add II (16.80g), benzene, BF 3 AcOH, reflux and stir for 30min, cool down in an ice-water bath, add water to quench the strong stirring, separate the liquids, wash the organic phase with water, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a tan solid (16.20 g).
Embodiment 2
[0041] 3-Ethoxy-3,5,9(11)-androstatriene-3,17-dione (IV)
[0042] Under nitrogen protection, add III (8.00g), THF, triethyl orthoformate, TsOH·H 2 O, stirred and reacted for 2h, added a small amount of triethylamine to the reaction solution, then added saturated aqueous sodium bicarbonate solution and stirred vigorously, extracted with ethyl acetate, washed the combined organic phase with water, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a reddish-brown solid (9.01g).
Embodiment 3
[0044] 3-keto-4,9(11)-diene-17β-hydroxypregna-21-carboxylate γ-lactone (V)
[0045] Install a dropping funnel and a nitrogen inlet tube on the 100mL three-necked bottle. Add 19 mL of dry dimethyl sulfoxide (DMSO) to the reaction flask, add 60% sodium hydride, and heat to 70° C. for 1 hour after the addition is complete. The cooled reaction solution was diluted with dry THF. Subsequently, the temperature of the reaction system was lowered to about -5°C, and then a solution of trimethylsulfide iodide dissolved in dry dimethyl sulfoxide was added dropwise. After stirring for a few minutes, a solution of 2.7 g of IV dissolved in THF was added dropwise. During the dropwise addition, keep the system temperature not higher than 0°C. After the dropwise addition was completed, the reaction was stirred at 0° C. for 2 hours and then raised to room temperature for 15 hours. The reaction was quenched with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, spin-dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com