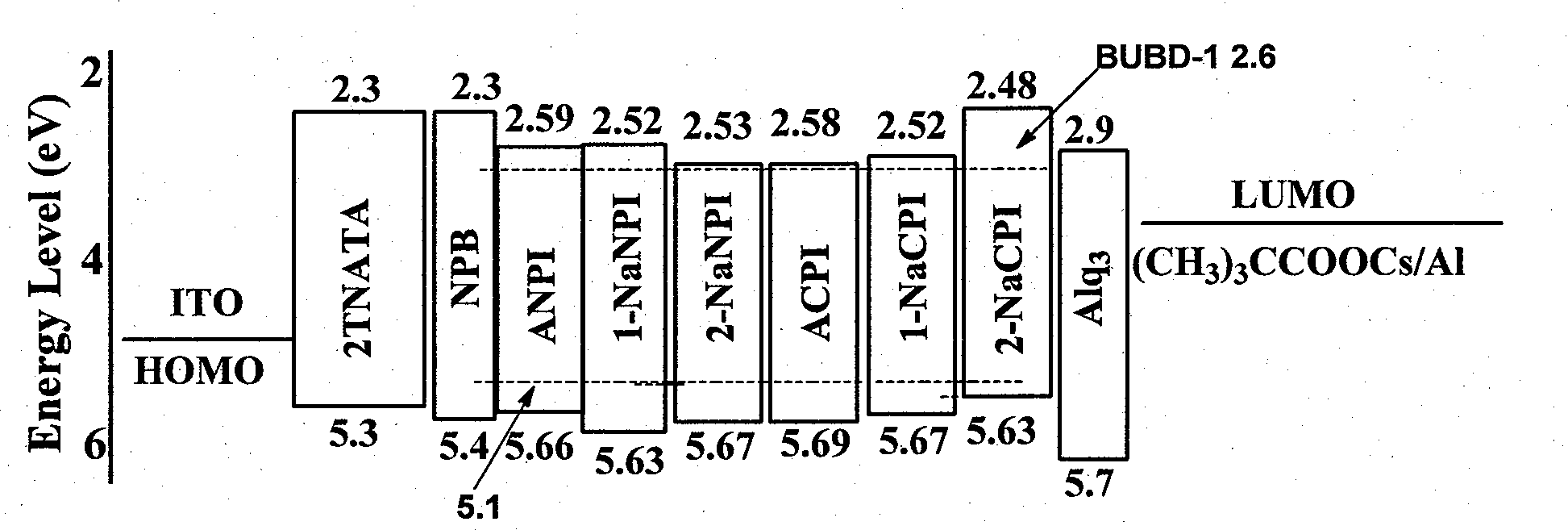
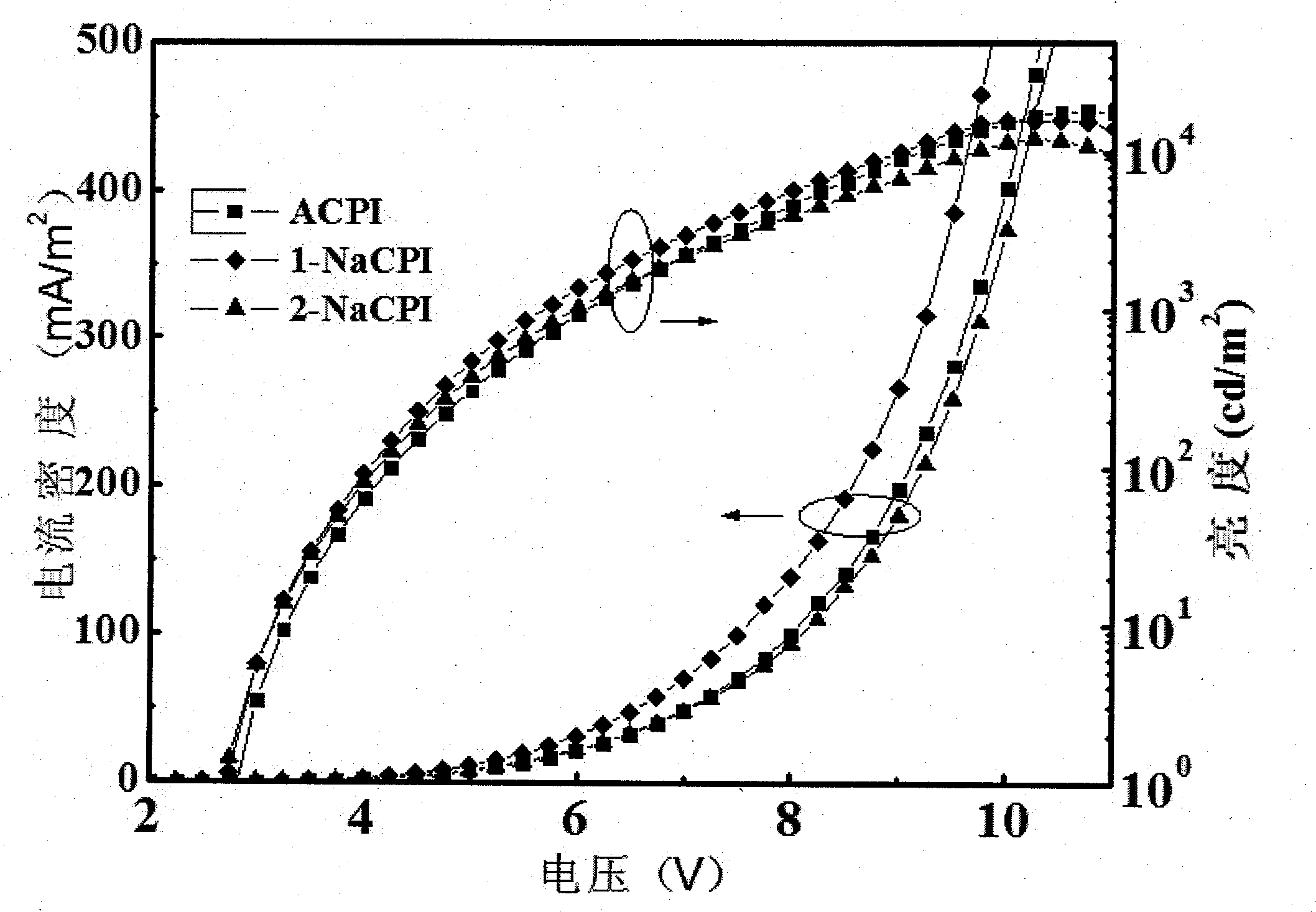
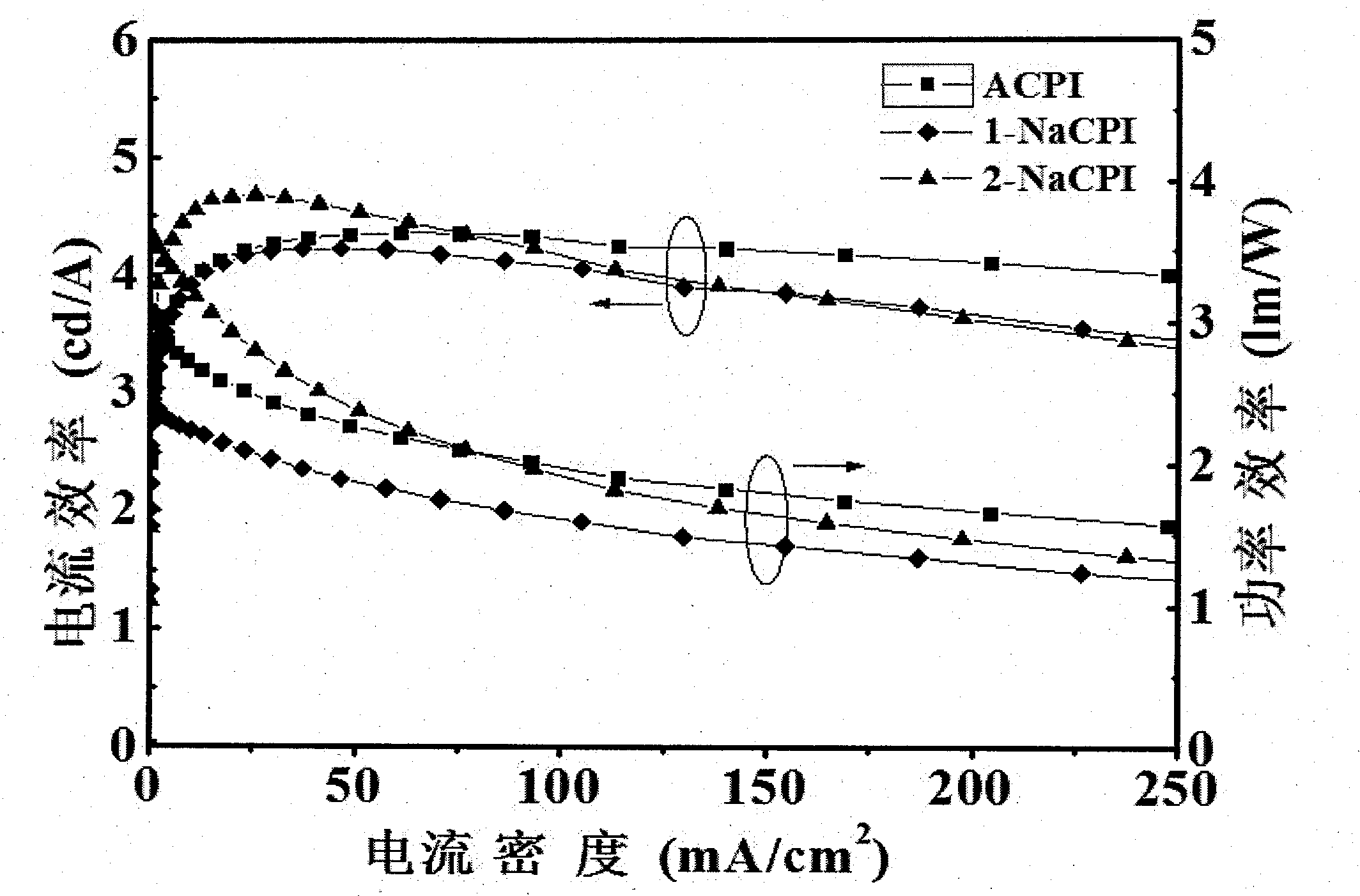
Phenanthroimidazole derivative and its application as electroluminescent material
A technology of phenanthroimidazole and electroluminescent devices, which is applied in the direction of luminescent materials, circuits, electrical components, etc., can solve the problems of low efficiency, unsatisfactory molecular efficiency, light color, poor material stability, etc., and achieve high glass transition temperature and good electronic properties. Good transmission ability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation method of ACPI of the present invention, this method comprises following three steps:
[0033] (1) Add 208 mg of dry phenanthrenequinone, 93.1 mg of aniline, 185 mg of p-bromobenzaldehyde, and acetic acid as a solvent in the reaction flask, react at 130°C for 12 hours, add 50 mL of water after cooling, filter the precipitate, wash with water, and dry to obtain the product 2- (4-Bromophenyl)-1-phenylphenanthroimidazole 360 mg, yield 80%.
[0034]Dissolve 360 mg of 2-(4-bromophenyl)-1-phenylphenanthroimidazole in 50 mL of anhydrous THF, inject 1.1 molar ratio of n-butyllithium at -78 ° C, and inject 1.5 molar ratio of n-butyl lithium after reacting for 1 hour. Anhydrous trimethyl borate, reacted for 12 hours, added water to quench the reaction, extracted with dichloromethane, dried, and recrystallized from n-hexane to obtain 242mg of 4-(1-phenyl-2-p-phenylphenanthroimidazolyl)phenylboronic acid , yield 73%.
[0035] 4-(1-phenyl-2-p-phen...
Embodiment 2
[0037] Example 2: 1-NaCPI described in the present invention can be synthesized by the following method.
[0038] (1) Add 208 mg of dry phenanthrenequinone, 93.1 mg of aniline, 185 mg of p-bromobenzaldehyde, and acetic acid as a solvent in the reaction flask, react at 130°C for 12 hours, add 50 mL of water after cooling, filter the precipitate, wash with water, and dry to obtain the product 2- (4-Bromophenyl)-1-phenylphenanthroimidazole 360 mg, yield 80%.
[0039] Dissolve 360 mg of 2-(4-bromophenyl)-1-phenylphenanthroimidazole in 50 mL of anhydrous THF, inject 1.1 molar ratio of n-butyllithium at -78 ° C, and inject 1.5 molar ratio of n-butyl lithium after reacting for 1 hour. Anhydrous trimethyl borate, reacted for 12 hours, added water to quench the reaction, extracted with dichloromethane, dried, and recrystallized from n-hexane to obtain 242mg of 4-(1-phenyl-2-p-phenylphenanthroimidazolyl)phenylboronic acid , yield 73%.
[0040] 4-(1-phenyl-2-p-phenylphenanthroimida...
Embodiment 3
[0041] Example 3: 2-NaCPI described in the present invention can be synthesized by the following method.
[0042] (1) Add 208 mg of dry phenanthrenequinone, 93.1 mg of aniline, 185 mg of p-bromobenzaldehyde, and acetic acid as a solvent in the reaction flask, react at 130°C for 12 hours, add 50 mL of water after cooling, filter the precipitate, wash with water, and dry to obtain the product 2- (4-Bromophenyl)-1-phenylphenanthroimidazole 360 mg, yield 80%.
[0043] Dissolve 360 mg of 2-(4-bromophenyl)-1-phenylphenanthroimidazole in 50 mL of anhydrous THF, inject 1.1 molar ratio of n-butyllithium at -78 ° C, and inject 1.5 molar ratio of n-butyl lithium after reacting for 1 hour. Anhydrous trimethyl borate, reacted for 12 hours, added water to quench the reaction, extracted with dichloromethane, dried, and recrystallized from n-hexane to obtain 242mg of 4-(1-phenyl-2-p-phenylphenanthroimidazolyl)phenylboronic acid , yield 73%.
[0044] 4-(1-phenyl-2-p-phenylphenanthroimida...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com