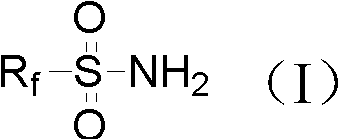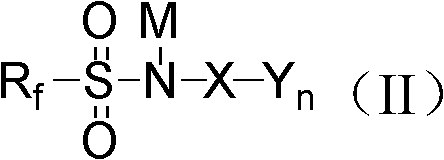Synthesis methods of alkali metal salt containing sulfonyl chloride or phosphorus imide and alkali metal salt containing fluorine sulfonyl or phosphorus imide
A technology of phosphoroimide base and fluorine-containing sulfonyl, which is applied in the field of preparation of fluorine compounds, and can solve the problems of high price of fluorine-containing acid anhydrides, large power consumption, and low atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of trifluoromethanesulfonamide:
[0072] The reaction formula is as follows:
[0073]
[0074] Add 156g (1mol) sodium trifluoromethanesulfinate (CF 3 SO 2 Na), 500mL water, 113g hydroxylamine oxysulfonic acid (NH 2 OSO 3 H) (1mol), 82g (1mol) sodium acetate (CH 3 COONa) was used as a buffer, and the reaction temperature was controlled by an ice-salt bath to be -10°C. After 10 hours of reaction, the reaction was stopped. Add an appropriate amount of sulfuric acid to adjust the pH of the system to 2-3. Add 100mL ether to extract, repeat twice. After the organic phases were combined, anhydrous sodium sulfate was added to dry, and ether was removed by rotary evaporation to obtain 104 g of a white solid product with a yield of 70%.
Embodiment 10
[0080] Preparation of (fluorosulfonyl) (trifluoromethylsulfonyl)imide potassium salt:
[0081]
[0082] In a 500mL three-necked flask, add 94g (0.5mol) trifluoromethanesulfonamide potassium salt, 80g (0.5mol) [(CH 3 ) 3 Si] 2 NH, 200mL 1,4-dioxane, heated to 145°C and refluxed for 12h. After completion of the reaction, the remaining [(CH 3 ) 3 Si] 2 NH and 1,4-dioxane to give the off-white solid product [CF 3 SO 2 ]NK[(CH 3 ) 3 Si] 2 . Then add 200mL 1,4-dioxane, dropwise add SO at room temperature 2 Cl 2 (0.5mol), after the dropwise addition, heat up to 80°C and reflux for 8h, distill off the by-product trimethylchlorosilane, add anhydrous 58g (1mol) KF, reflux at 80°C for 8h, suction filter, concentrate the filtrate, add etc. volume of CH 2 Cl 2 Perform recrystallization. After filtration, washing and drying, 77 g (0.35 mol) of a colorless crystalline solid of potassium salt of (fluorosulfonyl)(trifluoromethylsulfonyl)imide was obtained, with a yield of 78...
Embodiment 11
[0084] Preparation of (fluorophosphoryl) (trifluoromethylsulfonyl)imide potassium salt:
[0085]
[0086] To the 500mL flask, add 94g (0.5mol) CF 3 SO 2 NKSi(CH 3 ) 3 , 200mL nitromethane, phosphorus oxychloride was added dropwise at room temperature, after the dropwise addition was completed, reflux at 80°C for 10 hours. After completion of the reaction, the by-product trimethylchlorosilane was steamed, and the reaction system was transferred to a 500mL PFA bottle, and (C 2 h 5 ) 3 N(HF) 4 , Stirring for 4h at 40°C to stop the reaction. Use dry nitrogen flow at 50°C to remove excess residual hydrogen chloride and other volatile components, add saturated potassium carbonate solid under stirring at -30°C until there is no CO in the solution 2 Gas generation, add diethyl carbonate for extraction three times, 100mL each time, collect the diethyl carbonate phase, add 25g potassium carbonate to dry, filter, collect the filtrate, evaporate the solvent under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com