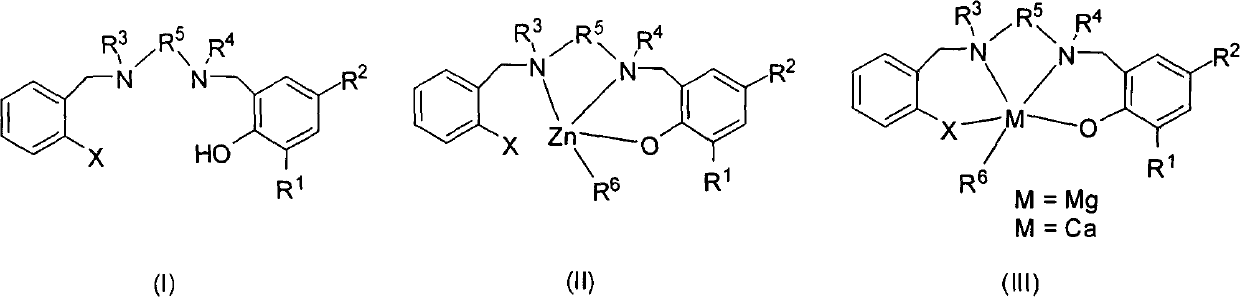
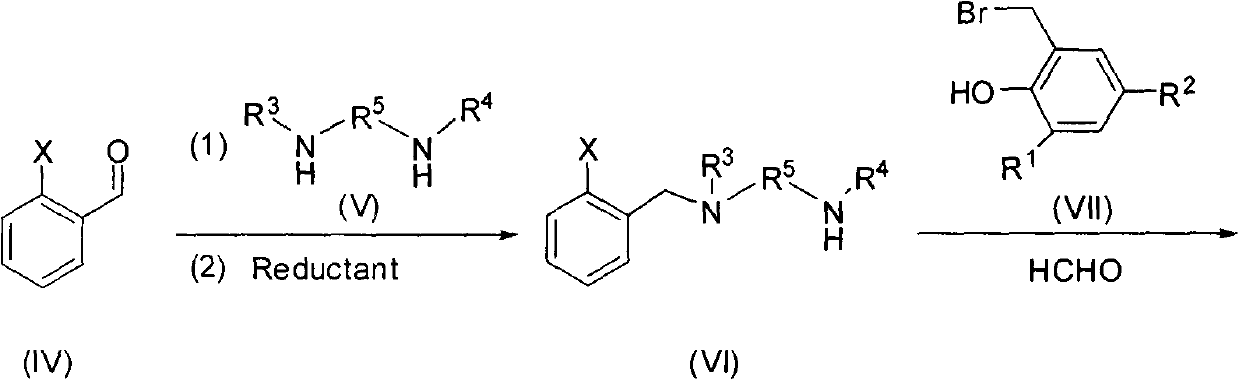
Similar salan monophenol ligand metal complexes as well as preparation method and application thereof
A technology of complexes and monophenols, applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, magnesium organic compounds, etc., can solve the problem of insufficient activity, low activity, inability to coordinate monomers and non-selectivity of insertion, etc. problems, to achieve the effect of convenient preparation, high catalytic activity and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] synthetic ligand L1
[0041]
[0042] Add 2.72g of 2-methoxybenzaldehyde, 20mL of anhydrous methanol, and 1.94g of N,N'-dimethylethylenediamine into a 100mL three-necked flask, and heat to reflux for 24h. Add 1.52g of sodium borohydride, heat to 50°C, add water to it, extract with dichloromethane, combine the organic phases, and dry with anhydrous magnesium sulfate, remove the solvent to obtain a light yellow viscous liquid, add 20mL of anhydrous Toluene and 1.34g dry KOH were added dropwise to a toluene solution of 5.12g 3,5-dichloro-2-benzylbromophenol, refluxed at 70°C, filtered to remove KOH, and the crude product was separated by column chromatography on silica gel to obtain ligand L1 (2.49 g, 22.07%).
[0043] 1 H NMR (CDCl 3 , 400MHz): δ7.32 (dd, 1H, J 1 =7.2Hz,J 2 =1.6Hz, ArH), 7.26-7.21(m, 2H, ArH), 6.90(td, 1H, J 1 =7.2Hz,J 2 =0.8Hz, ArH), 6.87-6.83(m, 2H, ArH), 3.78(s, 3H, OCH 3 ), 3.62(s, 2H, Ar-CH 2 N), 3.57(s, 2H, NCH 2 -Ar), 2.69-2.64 (m, 2H,...
Embodiment 2
[0045] Synthetic Ligand L2
[0046]
[0047] Add 2.72g of 2-methoxybenzaldehyde, 20mL of anhydrous methanol, and 1.94g of N,N'-dimethylethylenediamine into a 100mL three-necked flask, and heat to reflux for 24h. Add 1.52g of sodium borohydride, heat to 50°C, add water to it, extract with dichloromethane, combine the organic phases, and dry with anhydrous magnesium sulfate, remove the solvent to obtain a light yellow viscous liquid, add 20mL of anhydrous Toluene and 1.34g dry KOH were added dropwise to a toluene solution of 6.05g 3,5-dichloro-2-benzylbromophenol, refluxed at 70°C, filtered to remove KOH, and the crude product was separated by column chromatography on silica gel to obtain ligand L2 (1.94 g, 22.07%).
[0048] 1 H NMR (CDCl 3 , 400MHz): δ7.36(d, 1H, J=7.6Hz, ArH), 7.21(t, 1H, J=7.6Hz, ArH), 7.19(s, 1H, ArH), 6.91(t, 1H, J =8.0Hz, ArH), 6.84(d, 1H, J=8.0Hz, ArH), 6.80(s, 1H, ArH), 3.78(s, 3H, OCH 3 ), 3.68(s, 2H, Ar-CH 2 -N), 3.53(s, 2H, N-CH 2 -Ar), 2.61...
Embodiment 3
[0050] synthetic ligand L3
[0051]
[0052] Add 2.72g of 2-methoxybenzaldehyde, 20mL of anhydrous methanol, and 1.94g of N,N'-dimethylethylenediamine into a 100mL three-necked flask, and heat to reflux for 24h. Add 1.52g of sodium borohydride, heat to 50°C, add water to it, extract with dichloromethane, combine the organic phases, and dry with anhydrous magnesium sulfate, remove the solvent to obtain a light yellow viscous liquid, add 20mL of anhydrous Toluene and 1.34g dry KOH were added dropwise to a toluene solution of 8.81g 3,5-dicumyl-2-benzylbromophenol, refluxed at 70°C, KOH was removed by filtration, and the crude product was separated by column chromatography on silica gel to obtain the ligand L3 (4.96 g, 45.07%).
[0053] 1 H NMR (CDCl 3 , 400MHz): δ7.28-7.29(m, 5H, ArH), 7.17-7.25(m, 7H, ArH), 7.12(t, 1H, J=6.4Hz, ArH), 6.92(t, 1H, J= 7.4Hz, ArH), 6.86(d, 1H, J=8.0Hz, ArH), 6.73(s, 1H, ArH), 3.78(s, 3H, OCH 3 ), 3.57(s, 2H, Ar-CH 2 -N), 3.45(s, 2H, N-CH 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com