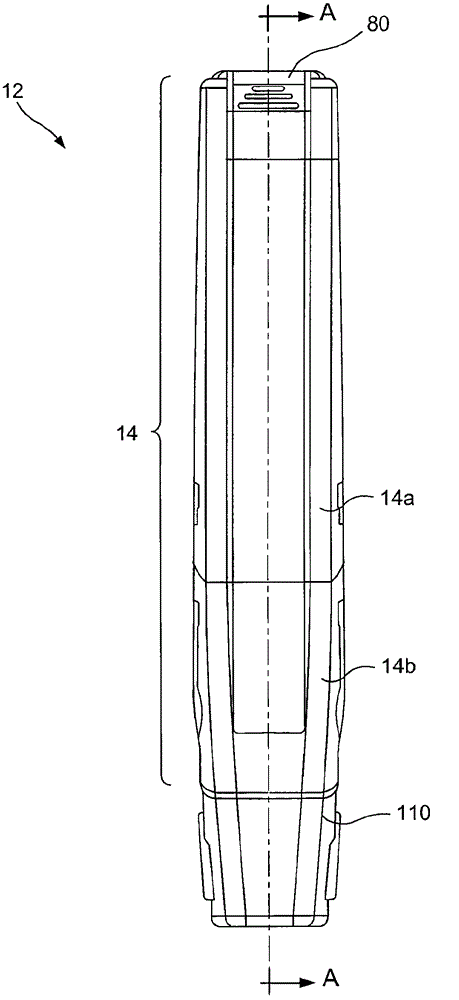
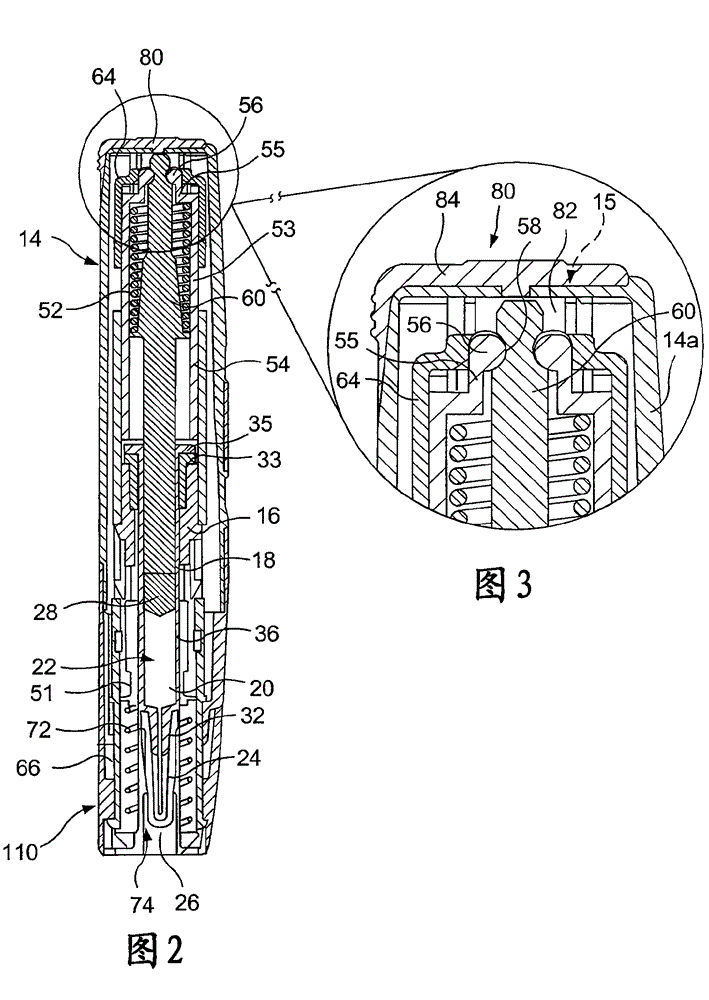
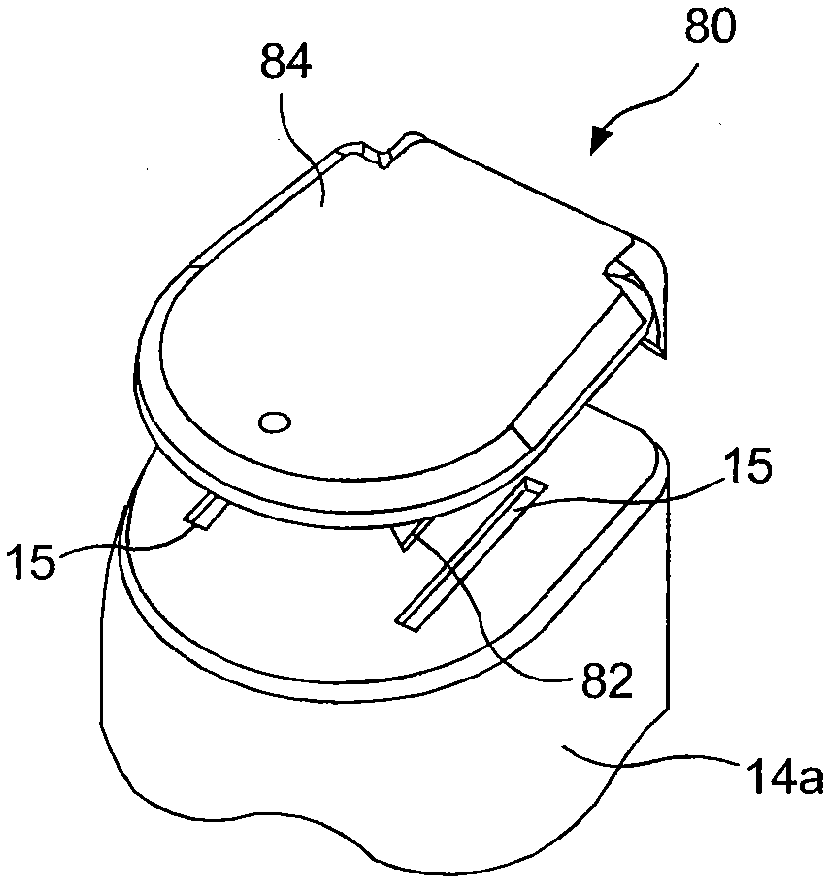
Hazardous Reagent Injection System
A technology for injectors and syringes, applied in the direction of syringes, anti-inflammatory agents, auto-injectors, etc., can solve problems such as impracticality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] Described and compared the pharmacokinetics of systemic exposure to the hazardous agent methotrexate achieved in male and female Göttingen minipigs following subcutaneous administration with the automatic injector of the present disclosure or with known hypodermic needle / syringe combinations. Kinetic (pharmacokinetic, PK) analysis.
[0211] Methotrexate and controls were administered. Test and control article administration, blood collection and processing for this study were performed at Charles River Preclinical Services (Spencerville, OH) under non-FDA compliant GLP (Good Laboratory Practice) conditions. The presented plasma concentration data were generated by a research grade Level 3 liquid chromatography tandem mass spectrometry (LC-MS / MS) method.
[0212] Methotrexate was administered subcutaneously in minipigs, and the same group of animals was injected with either an autoinjector or a needle / syringe. Injections were performed on days 1 and 8. Table 3 shows t...
Embodiment 2
[0249] The automatic injector of this disclosure and two known traditional automatic injectors Enbrel SureClick TM Autoinjector (Immunex Corporation, Thousand Oaks, CA, U.S.A.) and HUMIRA A comparison of injections between pens (Abbott Laboratories, Abbott Park, IL, U.S.A.) compared spring force and time required to deliver a full injection of 0.8-1.0 ml of solution.
[0250] A control was administered for this test. The results of two known automatic injectors were averaged. The results of the comparison are shown in Figure 16 . As shown in FIG. 6 , the time required to deliver the full injection is 10 seconds for the known automatic injector, but only 3 seconds for the automatic injector of the present disclosure. Therefore, users of known auto-injectors must hold these auto-injectors at the injection site for the full 10 seconds in order to receive the entire injection. In contrast, the user of the auto-injector of the present disclosure need only hold the auto-in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| penetration depth | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| penetration depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com