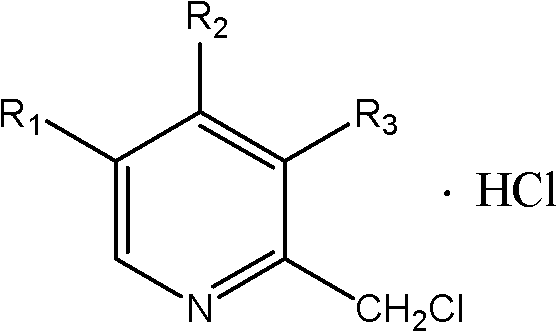
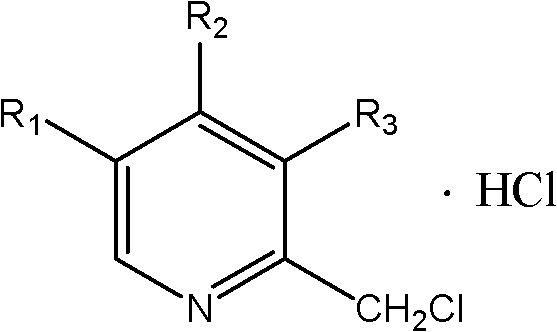
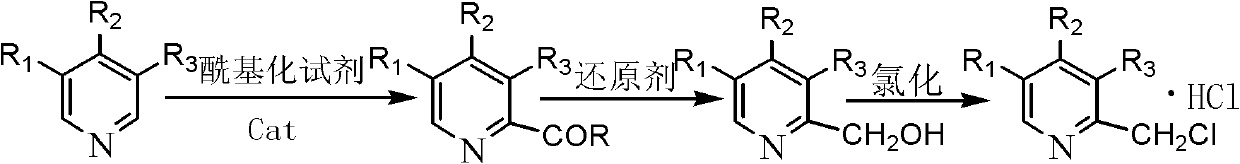
Synthetic method of chloromethylpyridine or pyridine derivative hydrochloride of chloromethylpyridine
A technology of chloromethylpyridine and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of troublesome recovery of precious metals, high pressure on cost and price, and dangerous operation of feeding materials, and achieve the effects of low cost, few reaction steps, and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500ml flask, add 9.2g of 4-methoxy-3,5-lutidine nitrogen oxide, 60ml of methanol, add 0.8g of aluminum trichloride, cool to below 0°C and start adding ethyl chloroformate dropwise After the addition of 7.8g of ester, the temperature was raised to a reaction temperature of 45°C to 95°C, and the reaction was carried out under reflux for 4 to 6 hours. Sampling and analysis was carried out until the raw material 4-methoxy-3,5-lutidine nitrogen oxide disappeared. until reduced; add 6.75g of sodium tetrahydroborate to the system, and the reaction temperature is 55-65°C for reduction. React for 5 hours, filter, filter out inorganic salts, distill the filtrate to remove methanol, extract 2-hydroxymethyl-4-methoxy-3,5-lutidine with dichloromethane, dry the dichloromethane phase, and cool 14g of thionyl chloride was added dropwise at -10°C, and then the temperature was naturally raised to room temperature for 2-3 hours to obtain 2-chloromethyl-4-methoxy-3,5-lutidine hydrochl...
Embodiment 2
[0022] In a 500ml flask, add 10g of 4-nitro-3,5-lutidine nitrogen oxide, 60ml of methanol, add 0.5g of magnesium oxide, cool to below 0°C and start adding 6.2g of acetic anhydride dropwise, dropwise After completion, raise the temperature to a reaction temperature of 45°C to 95°C, reflux for 4 to 6 hours, and take samples for analysis until the raw material 4-nitro-3,5-lutidine nitrogen oxide does not decrease; add 6.75g of lithium tetrahydrogen aluminum, the reaction temperature is 85-95 ° C, for reduction. React for 7 hours, filter, filter out inorganic salts, distill the filtrate to remove methanol, extract 2-hydroxymethyl-4-nitro-3,5-lutidine with dichloromethane, dry the dichloromethane phase, and cool to 18g of phosphorus trichloride was added dropwise at -20°C, and then the temperature was naturally raised to room temperature for 2-3 hours to obtain 2-chloromethyl-4-nitro-3,5-lutidine hydrochloride, which was weighed 11.9 g of off-white solid was obtained, and analyzed...
Embodiment 3
[0024] In a 500ml flask, add 7.3g of 3,5-lutidine nitrogen oxide, 60ml of methanol, add 4.0g of zinc chloride, cool to below 0°C and start adding chloroacetyl (CH 3 COCl) 9.3g, after the dropwise addition is completed, the temperature is raised to a reaction temperature of 45°C to 95°C, and the reflux reaction is carried out for 4 to 6 hours, and sampling and analysis are carried out until the raw material 3,5-lutidine nitrogen oxide does not decrease; Add 6.75gFe / 10mlAcOH to it, and the reaction temperature is 45-55°C for reduction. React for 12 hours, filter out inorganic salts, distill off methanol, extract 2-hydroxymethyl-3,5-lutidine with dichloromethane, dry the dichloromethane phase, cool to -5°C and start feeding 8.4g Chlorine, and then naturally warmed up to room temperature for 1 to 2 hours to obtain 2-chloromethyl-3,5-lutidine hydrochloride. Weighed 9.1 g of white solid, analyzed by HPLC: the purity was 98.45%. The total yield of the three-step reaction is 80.5%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com