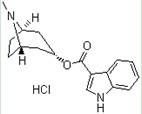
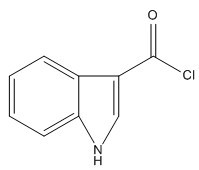
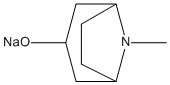
Method for preparing tropisetron hydrochloride on large scale
A technology of tropisetron hydrochloride and tropisetron, which is applied in the direction of organic chemistry, can solve the problems of high price, and achieve the effects of simple operation, cost saving and increased safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] (1) Preparation of indole-2-carbonyl chloride:
[0025] Add 20.0g of indole-3-carboxylic acid, 300mL of 1,2-dichloroethane and 3 mL of DMF into a 500mL three-neck flask, slowly add 20 mL of thionyl chloride dropwise under stirring, react at 45°C for 8h, and evaporate excess Thionyl chloride and 1,2-dichloroethane were dissolved in 100 mL tetrahydrofuran for later use.
[0026] (2) Preparation of α-tropine sodium:
[0027] Add 19.0g of α-tropine alcohol, 100mL of tetrahydrofuran and 5.4g of NaOH solid into a 500 mL three-neck flask, stir and react at room temperature for 4h, and set aside.
[0028] (3) Preparation of Tropisetron Hydrochloride:
[0029] Slowly add the THF solution of indole-3-formyl chloride to the THF solution of sodium tropinate at room temperature, stir and react at 35°C overnight, recover THF by distillation under reduced pressure, and recrystallize with 95% ethanol to obtain a light yellow solid. Add 70 mL of absolute ethanol, heat to dissolve, pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com