Compound containing framework of chiral indolone and angelica lactone and asymmetric synthesis method
An angelica lactone and product technology, applied in the field of allyl alkylated compounds, can solve problems such as the limitation of reactant types, and achieve the effects of wide application range of substrates, good yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Allyl alkylation of indolinone MBH carbonate and various angelica lactone compounds catalyzed by chiral cinchona base-derived b-ICD derivatives
[0021] In a clean reaction tube, add chiral cinchona base-derived b-ICD derivative catalyst (0.01 mmol), indolinone MBH carbonate (0.10 mmol), angelica lactone compound (0.20 mmol), and Mesitylene 0.1 mL, at -10 o C for the corresponding time with stirring. The product was isolated by column chromatography.
[0022]
[0023] P1, 80% yield; [α] D 20 = +8.5 ( c = 0.4 in CHCl 3 ); 90% ee, chiral test conditions: determined by HPLC analysis [Daicel Chiralcel OD, n -Hexane / i -PrOH = 80 / 20, 1.0 mL / min, λ = 254 nm, t (major) = 26.532 min, t (minor) = 18.793 min,]; 1 H NMR (400 MHz, CDCl 3 ): δ = 7.26 (t, J = 7.6 Hz, 1H), 7.13 (d, J = 7.2 Hz, 1H), 7.06 (d, J = 5.6 Hz, 1H), 6.96 (t, J = 7.6 Hz, 1H), 6.84 (s, 1H), 6.79 (d, J = 7.6 Hz, 1H), 6.75 (s, 1H), 5.70 (d, J = 5.6 Hz, 1H), 3.62 (s, 3H), 3.29 (s,...
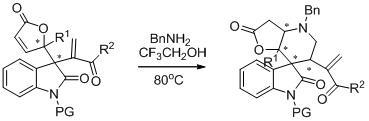
Embodiment 2
[0037] Example 2: Transformation with multifunctional functional groups (application example 1)
[0038]
[0039] P1 (65.4 mg, 0.20 mmol) and BnNH 2 (110 μL, 1.00 mmol) dissolved in CF 3 CH 2 OH (0.5 mL), 80 o C was stirred until P1 was detected by TLC and disappeared. Add Boc at room temperature 2 O (436 mg, 2.00 mmol) to remove unreacted BnNH 2 . Column chromatography (petroleum ether / ethyl acetate: 2 / 1) gave the title compound as a colorless oil. (61.0 mg, 70% yield). [α] D 20 = -9.2 ( c = 0.9 in CHCl 3 ); 90% ee, chiral test conditions: determined by HPLC analysis [Daicel Chiralcel AD, n -Hexane / i -PrOH = 70 / 30, 1.0 mL / min, λ = 254 nm, t (major) = 15.195 min, t (minor) = 14.046 min]; 1 H NMR (400 MHz, CDCl 3 ): δ = 7.54 (d, J = 7.2 Hz, 1H), 7.41-7.30 (m, 6H), 6.97 (td, J = 7.6 Hz, 0.8 Hz, 1H), 6.86 (d, J = 8.0 Hz, 1H), 3.97 (d, J = 13.6 Hz, 1H), 3.71 (dd, J = 7.2 Hz, 6.0 Hz, 1H), 3.66 (d, J = 13.6 Hz, 1H), 3.35 (d, J = 5.6 Hz, 1H), 3.29 (d...
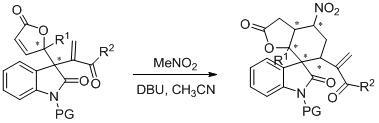
Embodiment 3
[0040] Example 3: Transformation with multifunctional functional groups (application example 2)
[0041]
[0042] P1 (50.0 mg, 0.15 mmol), MeNO 2 (1.50 mmol, 80 μL) and DBU (0.07 mmol, 11.4 mg) in CH 3 CN (0.5 mL), 70 o C was stirred until P1 was no longer converted as detected by thin layer chromatography. Column chromatography (petroleum ether / ethyl acetate: 2 / 1) gave a white solid. (23.0 mg, 40% yield). [α] D 20 = +5.5 ( c = 0.9 in CHCl 3 ); 90% ee, chiral test conditions: determined by HPLC analysis [Daicel Chiralcel OD, n -Hexane / i -PrOH = 60 / 40, 1.0 mL / min, λ = 254 nm, t (major) = 26.963 min, t (minor) = 19.554 min]; 1 H NMR (400 MHz, CDCl 3 ): δ = 7.42 (t, J = 7.6 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.12 (t, J = 7.6 Hz, 1H), 6.94 (d, J = 7.6 Hz, 1H), 4.76 (m, 1H), 3.56 (dd, J = 12.8 Hz, 5.6 Hz, 1H), 3.46 (s, 3H), 3.29 (s, 3H), 3.11 (dd, J = 10.8 Hz, 6.8 Hz, 1H), 2.86-2.80 (m, 2H), 2.97 (dd, J = 18.0 Hz, 6.8 Hz, 1H), 2.70 (d, J = 18.0 Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com