Preparation method for secondary alcohol
A secondary alcohol and compound technology, applied in the field of secondary alcohol preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3aa to 3a
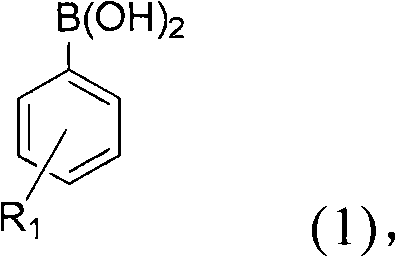
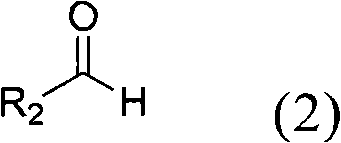
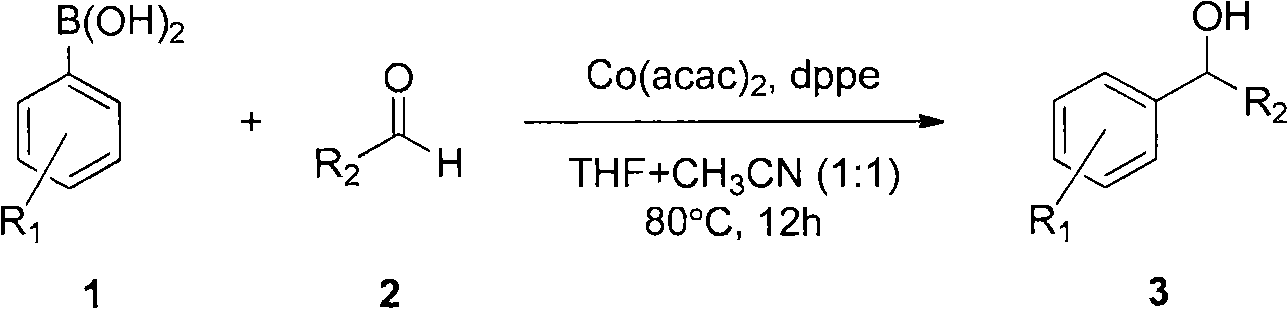
[0038] Among the above reaction conditions, phenylboronic acid (1a) reacts with aromatic aldehydes, heterocyclic aldehydes and aliphatic aldehydes with various substituents to produce secondary alcohols. Benzaldehyde with electron-withdrawing groups (for example, with 4-NO 2 Substituent (2b), with 4-CHO substituent (2c), with 4-CO 2 Me substituent (2d) and have 4-CF 3 Substituent (2e)) gave diarylcarbinols 3ab-3ae in good yields (89-97%; Table 1 entries 2-5).
[0039] Benzaldehyde derivatives with halogen substituents are also suitable for the catalytic reaction of the present invention. For example substituents 4-F(2f), 3-F(2g), 2-F(2h), 4-Cl(2i) and 4-Br(2j) can react efficiently with phenylboronic acid (1a) to generate relative The diarylmethanols 3af-3aj have good or excellent yields (please refer to Table 1 No. 6-10).
[0040] Similarly, benzaldehyde (2k), 1-napthalaldehyde (1-napthalaldehyde, 2l), and 2-napthalaldehyde (2-napthalaldehyde, 2m) can also undergo additio...
Embodiment 3bd-3id
[0045] In addition, arylboronic acids having various substituents in the present invention can be reacted with methyl 4-formylbenzoate (2d). The substituents of arylboronic acid include 4-Br(1b), 4-F(1c), 4-CHO(1d), 4-Me(1e), 4-OMe(1f), 2-OMe(1g) and 4 -vinyl(1h) can be reacted with 2d to produce substituted diaryl carbinols 3bd-3hd in yields of 93, 93, 84, 92, 97, 96 and 75% (refer to Table 1 No. 20- 26).
[0046] From the above results, it can be seen that the addition reaction of the present invention is widely applicable to various functional groups, such as Br, F, CHO, Me and OMe.
[0047] In addition, this catalytic reaction is also applicable to phenylboronic acids with alkenyl groups. Among them, (E)-styrylboronic acid ((E)-styrylboronic acid, 1i) was reacted with 2d to obtain a secondary alcohol with an allyl group, and the yield was 78% (please refer to No. 27 in Table 1).
Embodiment
[0048] Example: using different catalysts
[0049] in Co(acac) 2 (5mol%), dppe (5mol%) dissolved in THF / CH 3 In CN (1:1), phenylboronic acid (1a) and 4-cyanobenzaldehyde (2a) were reacted at 80° C. for 12 hours to obtain the addition product 3aa with a yield of 96%.
[0050] In this reaction, no additional base was needed, only 1.2 mmol of organoboronic acid was used.
[0051] The above catalytic reactions can also use CoI 2 or CoCl 2 (5mol%) and dppe (5mol%) as catalyst, and use THF as solvent to obtain 3aa, its yield is 9697%, but this time needs to add base K 2 CO 3 (1.5 equivalents) to activate boronic acid.
[0052] enantioselective secondary alcohols
[0053] Please refer to Table 2 and the following reaction formula, another object of the present invention provides a kind of enantioselective preparation method of secondary alcohol, it is by making an organic boronic acid compound 1 and an aldehyde compound 2 in an organic solution Reaction to obtain enantioselec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com