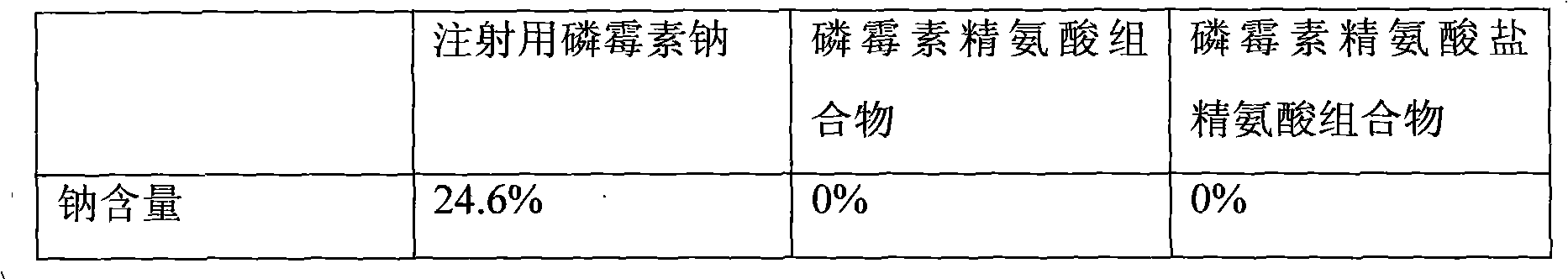
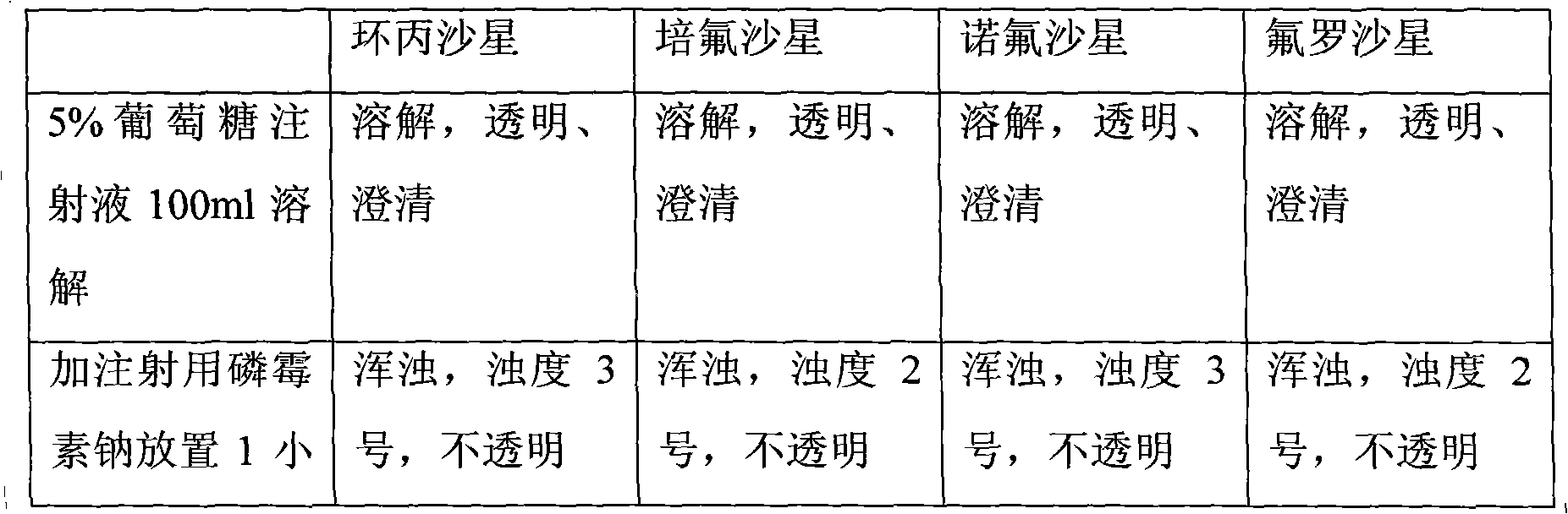
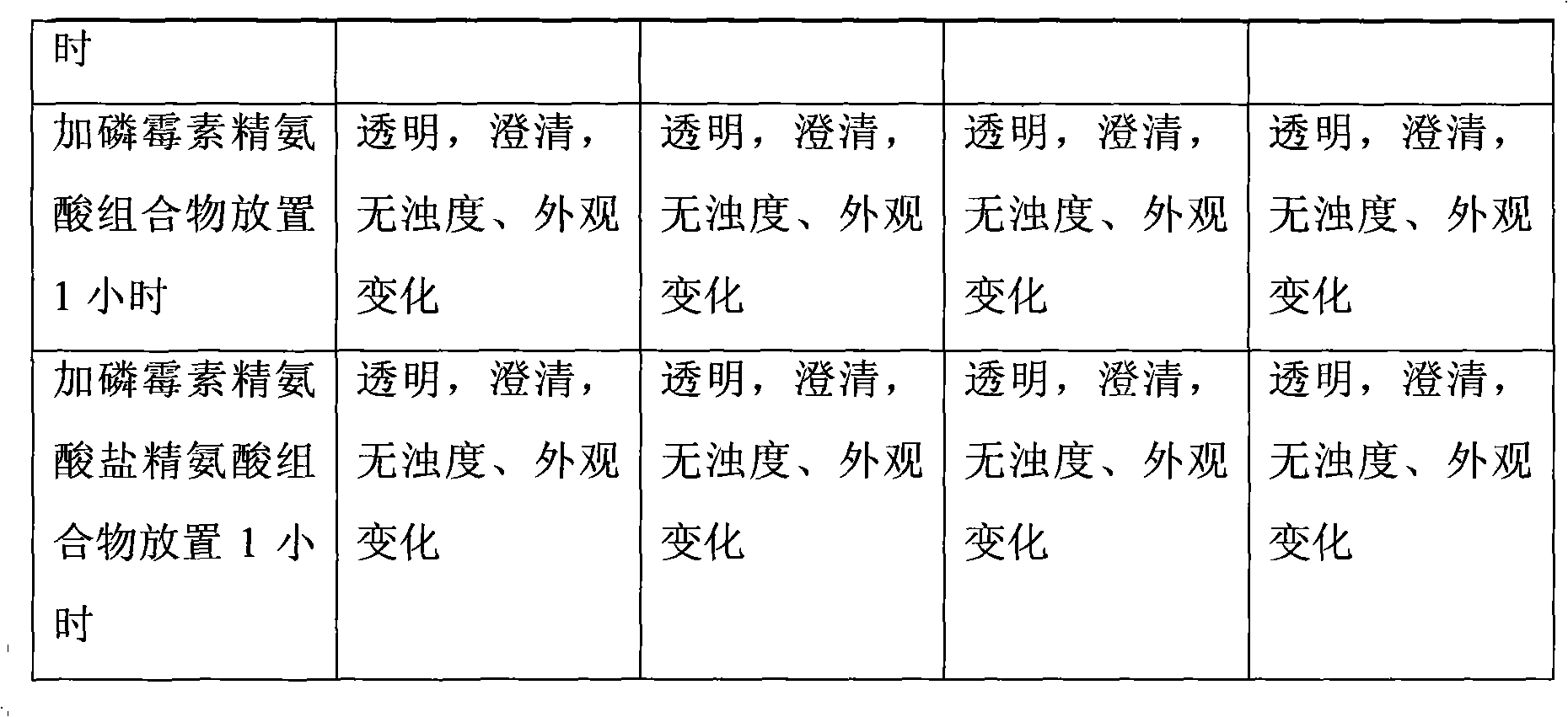
Composition of fosfomycin or fosfomycin arginine salt and arginine
A technology of arginine salt and composition, applied in the field of pharmaceutical preparation, to achieve the effects of wide application range, good fluidity and stability, and easy mixing and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of the fosfomycin arginine composition of ratio 1:
[0038] In a sterile environment, 1 kg of sterile fosfomycin and 2.5 kg of sterile arginine (both calculated as dry and pure) were passed through a 60-mesh sieve and mixed in a three-dimensional mixer at a speed of 100 revolutions per minute. Mix for 1.5 hours. After mixing evenly, discharge the material, put it into an aluminum bucket for antibiotic packaging, seal it with an aluminum cover, cover it with a polyethylene plastic bag, seal it, and pack it into a box.
[0039] After packing, it is transported to the aseptic preparation subpackaging workshop, the outer packaging is removed, and the aluminum barrel is sterilized by ultraviolet light, then enters the aseptic powder subpackaging line, and is aseptically subpackaged into antibiotic glass bottles according to the specifications to obtain the preparation of the composition of the present invention. finished product.
Embodiment 2
[0040] The preparation of the fosfomycin arginine composition of embodiment 2 ratio 2:
[0041] In a sterile environment, 2.0kg of sterile fosfomycin and 3.0kg of sterile arginine (both calculated as dry and pure) were passed through a 60-mesh sieve and mixed in a three-dimensional mixer at a speed of 100 rpm. , Mix for 1.5 hours. After mixing evenly, discharge the material, put it into an aluminum bucket for antibiotic packaging, seal it with an aluminum cover, cover it with a polyethylene plastic bag, seal it, and pack it into a box.
[0042] After packing, it is transported to the aseptic preparation subpackaging workshop, the outer packaging is removed, and the aluminum barrel is sterilized by ultraviolet light, then enters the aseptic powder subpackaging line, and is aseptically subpackaged into antibiotic glass bottles according to the specifications to obtain the preparation of the composition of the present invention. finished product.
Embodiment 3
[0043] The preparation of the fosfomycin arginine composition of embodiment 3 ratio 3:
[0044] In a sterile environment, 2.0kg of sterile fosfomycin and 4.0kg of sterile arginine (both calculated as dry and pure) were passed through a 60-mesh sieve and mixed in a three-dimensional mixer at a speed of 100 rpm. , Mix for 1.5 hours. After mixing evenly, discharge the material, put it into an aluminum bucket for antibiotic packaging, seal it with an aluminum cover, cover it with a polyethylene plastic bag, seal it, and pack it into a box.
[0045] After packing, it is transported to the aseptic preparation subpackaging workshop, the outer packaging is removed, and the aluminum barrel is sterilized by ultraviolet light, then enters the aseptic powder subpackaging line, and is aseptically subpackaged into antibiotic glass bottles according to the specifications to obtain the preparation of the composition of the present invention. finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com