(s) -2-benzyl-3- ( (3r, 4r) -4- (3 -carbamo ylphenyl) -3, 4-dimethylpiperidinyl) propanoic acid and salt therof as antagonists of the opioid receptors
An opioid receptor, IA-S-3 technology, applied in the direction of antitoxins, anti-inflammatory agents, non-central analgesics, etc., can solve the problems of lack of specificity and peripheral selective side effects of intestinal obstruction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
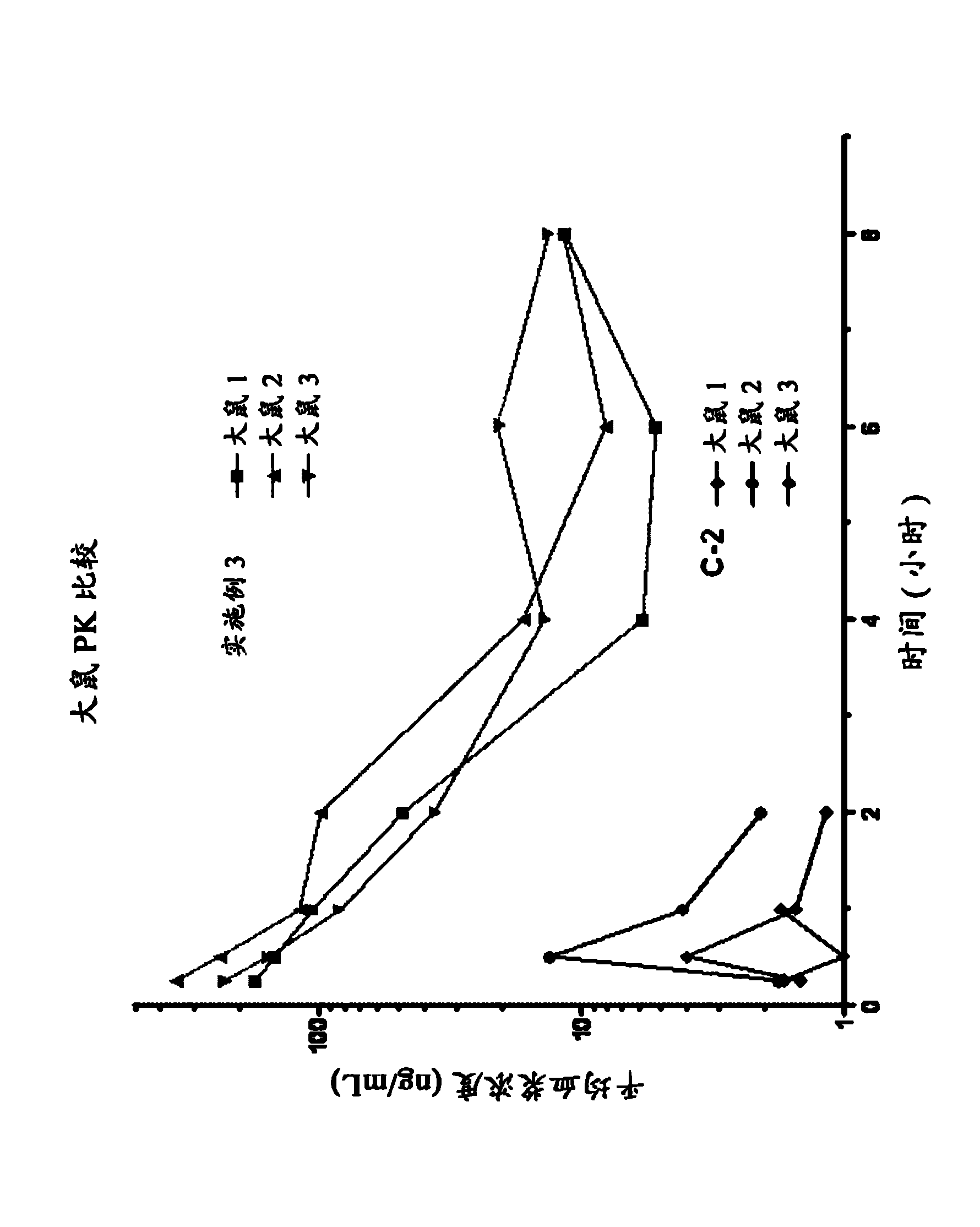
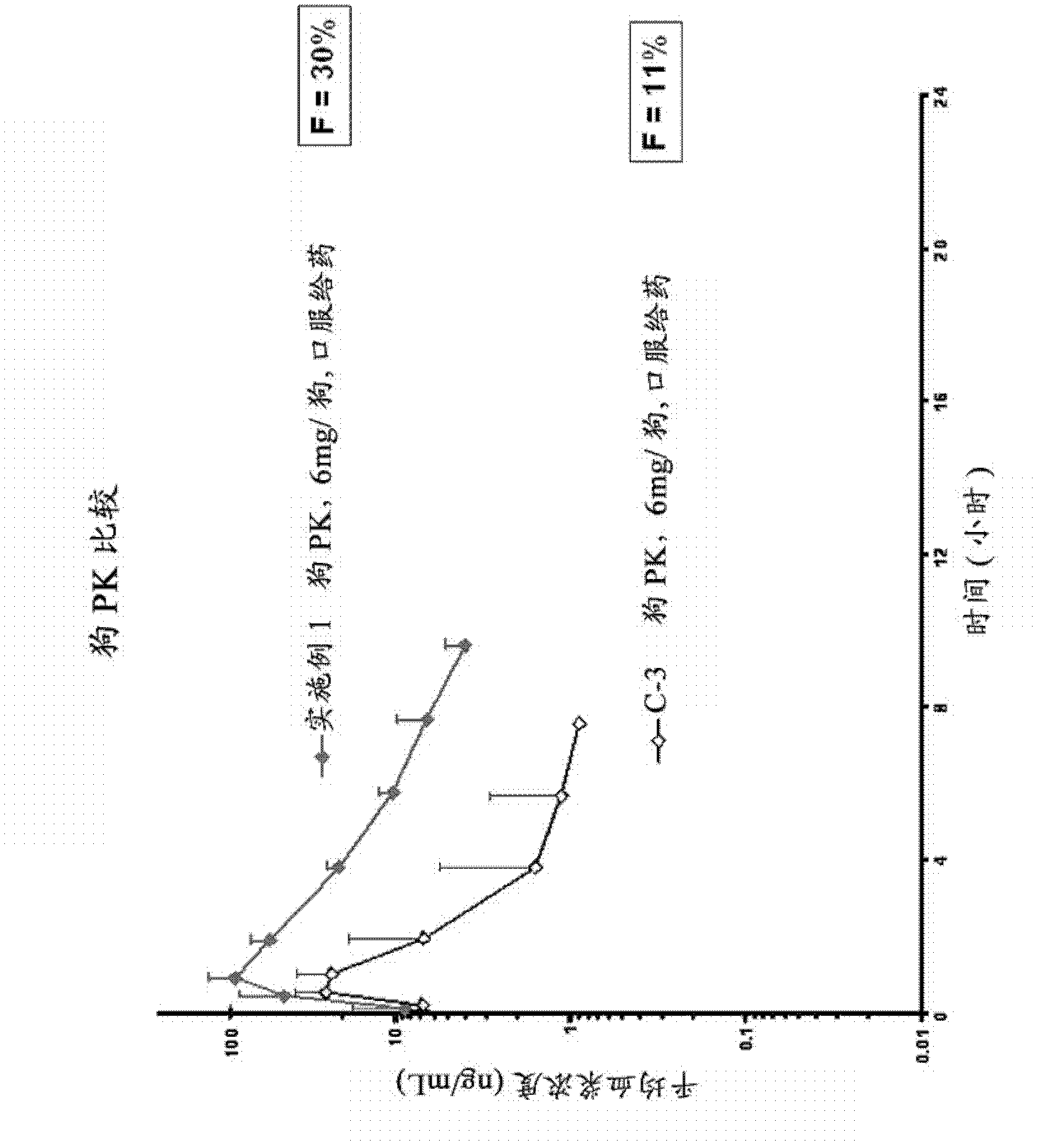
Image
Examples
Embodiment 1
[0138] Example 1: (S)-2-benzyl-3-((3R,4R)-4-(3-carbamoylphenyl)-3,4-dimethylpiperidin-1-yl)propionic acid Preparation of lithium(4a)
[0139] plan 1
[0140]
[0141] a.(S)-2-benzyl-3-((3R,4R)-3,4-dimethyl-4-(3-(trifluoromethyl-sulfonyloxy)phenyl)piperidine- 1-base) preparation of methyl propionate (2)
[0142]
[0143] To (S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propionic acid methyl ester (1) (5.00g, 0.0131mol) (see Werner et al., J.Org.Chem, 1996, 61, 587-597) in dichloromethane (70mL, 1mol) was added triethylamine (3.03mL, 0.0218 mol), then N-phenylbis(trifluoromethanesulfonimide) (7.02 g, 0.0196 mol) / dichloromethane (70 mL) was added dropwise. The mixture was stirred overnight at room temperature. LCMS indicated the reaction was complete. NaOH (1 N) was added and the mixture was stirred for 30 minutes. The resulting layers were separated and the aqueous layer was extracted with DCM. The combined organic layers were washed with N...
Embodiment 2
[0150] Example 2: (S)-2-benzyl-3-((3R,4R)-4-(3-carbamoylphenyl)-3,4-dimethylpiperidin-1-yl)propionic acid Preparation of sodium (4b)
[0151] Scenario 2
[0152]
[0153]
[0154] a.(S)-2-benzyl-3-((3R,4R)-3,4-dimethyl-4-(3-(trifluoromethyl-sulfonyloxy)phenyl)piperidine- 1-base) preparation of methyl propionate (2)
[0155] Example 1a was repeated except that the reaction was scaled up to use 40.0 g (0.1 mol) of (S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3 , methyl 4-dimethylpiperidin-1-yl]propionate (1) / dichloromethane (560mL), triethylamine (24.25mL, 0.174mol) and N-phenylbis(trifluoromethanesulfonyl imine) (56.2 g, 0.157 mol) / dichloromethane (70 mL). Obtained 50 g (93%) of (S)-2-benzyl-3-((3R,4R)-3,4-dimethyl-4-(3-(trifluoromethyl-sulfonyloxy)phenyl )piperidin-1-yl)methyl propionate (2) as a pale yellow oil. 1 H NMR (CDCl 3 ), δ0.68(d, J=7Hz, 3H), 1.29(s, 3H), 1.55(m, 1H), 1.96(m, 1H), 2.25(m, 1H), 2.39(m, 2H), 2.47(m, 1H), 2.68(m, 2H), 2.79(m, 2H), 2.93(m, 2...
Embodiment 3
[0160] Example 3: (S)-2-Benzyl-3-((3R,4R)-4-(3-carbamoylphenyl)-3,4-dimethylpiperidin-1-yl)propionic acid Preparation of Trifluoroacetate (4c)
[0161] Option 3
[0162]
[0163] a.(S)-tert-butyl 2-benzyl-3-((3R, 4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propionate ( 1a) Preparation
[0164] Di-tert-butoxy-N,N-dimethylmethylamine (30 mL, 0.1.mol, 4 equiv) was added dropwise to (S)-2-benzyl-3-((3R,4R)- 4-(3-Hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanoic acid (5) (see Werner et al., J.Org.Chem. 1996, 61, 587-597) (10.3 g, 0.028803 mol, 1 eq) in suspension in refluxing toluene (100 mL). The mixture was heated to reflux for 8 hours. The mixture was then cooled to room temperature, poured into 1N aqueous sodium hydroxide solution, and extracted. The crude product was purified by column chromatography (hexane / ethyl acetate 8:2) to give compound 1a (3.65 g, 31%). 1 H NMR (DMSO), δ0.65(d, J=7Hz, 3H), 1.20(s, 3H), 1.23(s, 9H), 1.48(d, J=13Hz, 1H), 1.92(dd, J= 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com