Achromobacter and method for asymmetrically catalytically reducing carbon-carbon double bond
A colorless bacillus, carbon-carbon double bond technology, applied in the field of biochemistry, to achieve the effect of easy preparation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 strain screening
[0026] Get 1 gram of soil sample and join into 50ml inorganic salt culture fluid (composition is as follows: KH 2 PO 4 0.1g / 100mL, Na 2 HPO 4 0.2g / 100mL, MgCl 2 0.04g / 100mL, NH 4 Cl 0.04g / 100mL), then add 100 μL of citral; another 1 gram of soil sample is added to 50ml of inorganic salt culture solution (the composition is as follows: KH 2 PO 4 0.1g / 100mL, Na 2 HPO 4 0.2g / 100mL, MgCl 2 0.04g / 100mL, NH 4Cl 0.04g / 100mL), add 100μL of (Z)-2-phenylbut-2-enenitrile; respectively enrich culture (30°C, 230rpm), take out 1mL each after 7 days under the same conditions The secondary enrichment culture was carried out for 8 days. Then add double distilled water 9mL to dilute and coat the plate, (the components of the plate medium are: tryptone 1g / 100mL; yeast extract 0.5g / 100mL; NaCl 1g / 100mL; agar powder 2g / 100mL, and each plate 100 μL each of citral or (Z)-2-phenylbut-2-enenitrile) was coated on the surface, cultured in a consta...
Embodiment 2
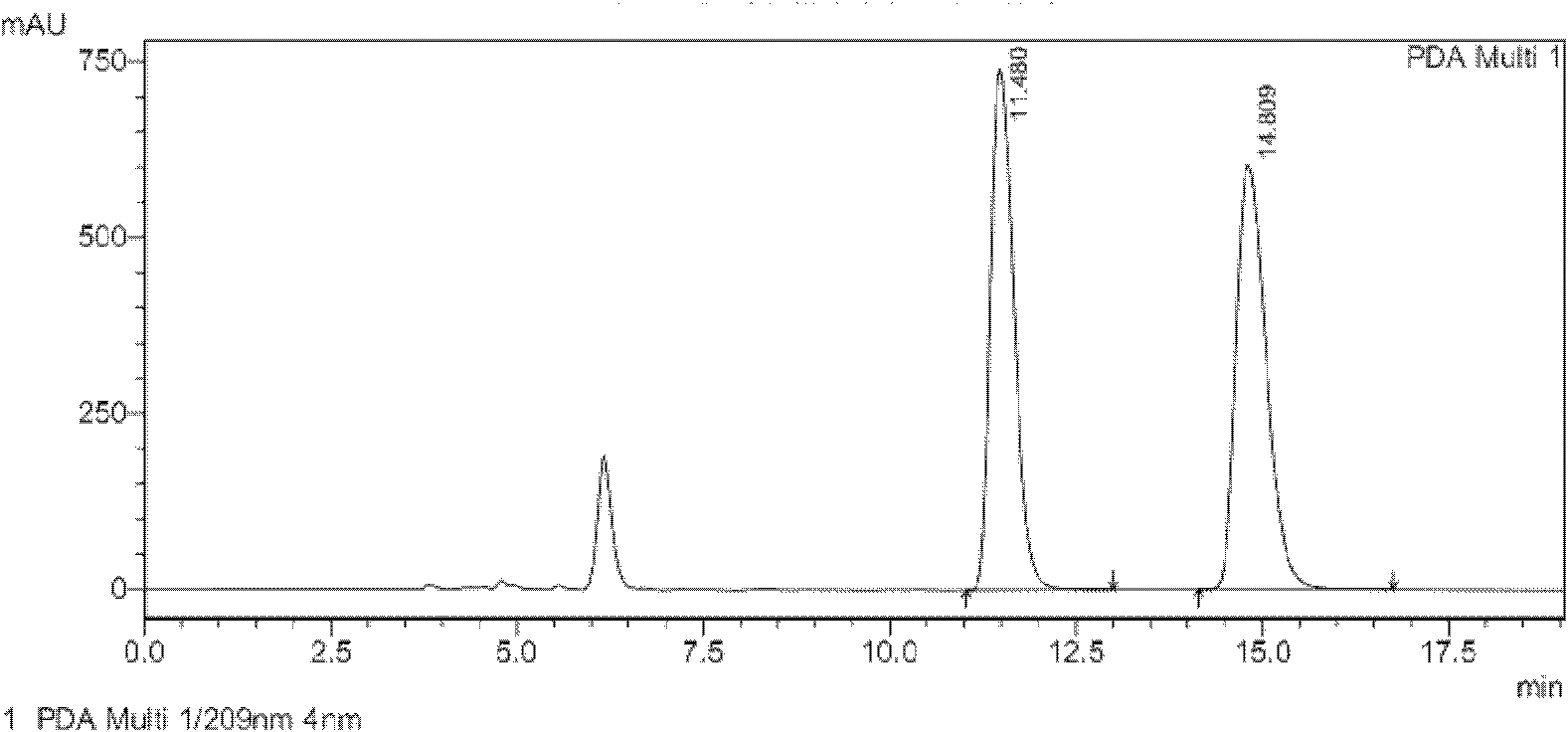
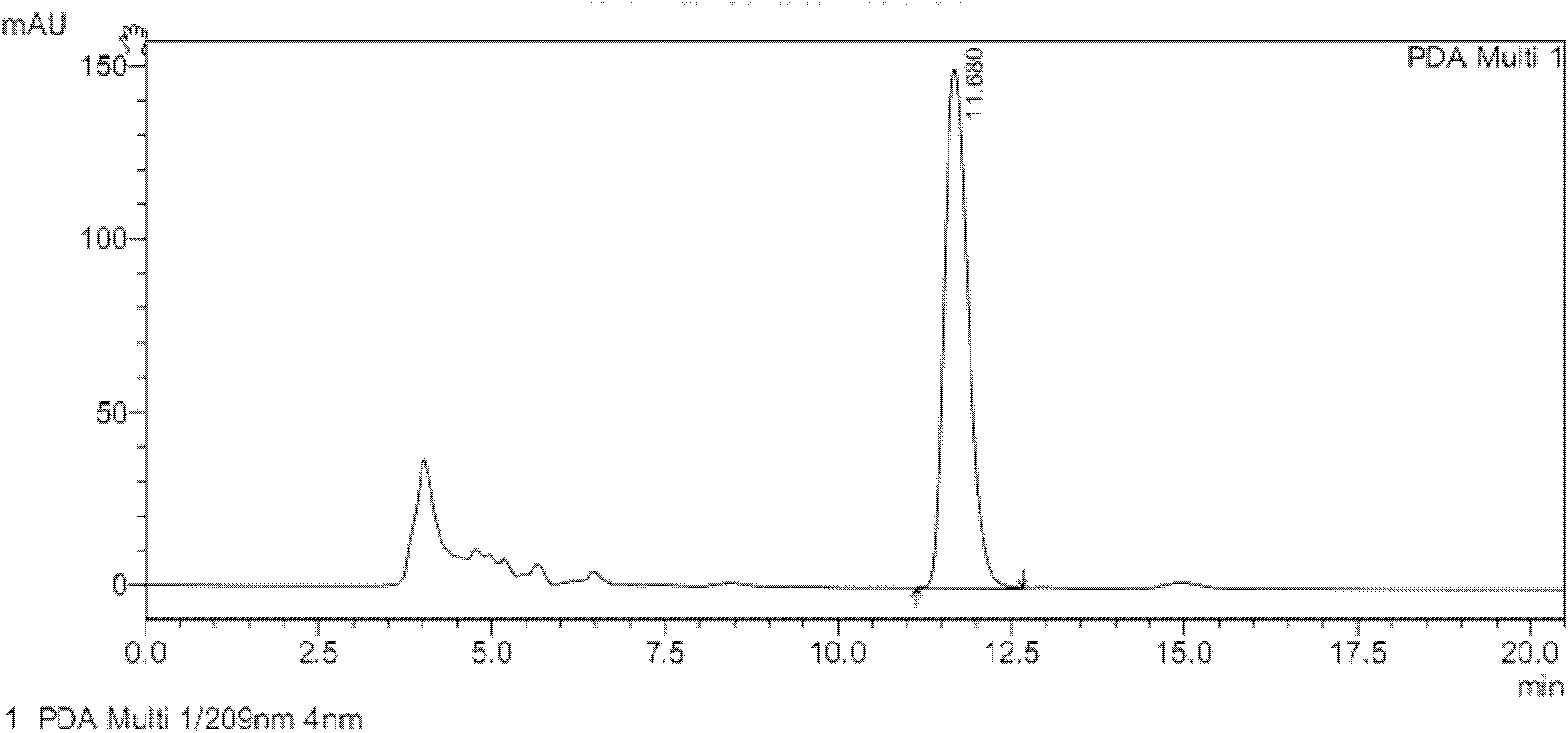
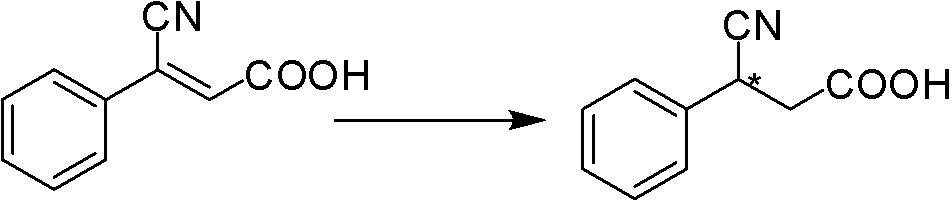
[0033] Conversion substrate (Z)-3-phenyl-3-cyano-acrylic acid during the screening of Example 2 Achromobacter JA81
[0034] The chiral 3-aryl-3-cyanopropionic acid catalyzed by JA81 is an intermediate in the synthesis of γ-aminobutyric acid. γ-Aminobutyric acid (GABA) is the most important inhibitory amino acid transmitter in the mammalian central nervous system (Fryszkowska et al.2010A short, chemoenzymatic route to chiral beta-aryl-gamma-amino acids using reductases from anaerobic bacteria.Org Biomol Chem 8:533-535). See the content of the invention in this manual for the cultivation method of the seeds and the composition of the culture medium. Take 1 g of freshly cultured wet bacteria, suspend in 10 mL of potassium phosphate buffer (0.1 M, pH 7.0), add 500 μL of isopropanol, 500 mg of glucose and 10 mg of (Z)-3-phenyl-3-cyano-acrylic acid ( The substrate is dissolved in dimethyl sulfoxide, and the dosage is 100g / L). Shake the reaction on a shaker (30°C, 230rpm) for 48 h...
Embodiment 3
[0035] The identification of embodiment 3 screening bacterial strains
[0036] In order to determine the taxonomic status of the strain, the strain was initially identified by 16S rRNA.
[0037] 16S rRNA identification: Genomic DNA was used as a template to amplify the 16S rRNA sequence with bacterial universal primers NS1 and NS8. Its sequence is as follows (1524bp):
[0038] AGAGTTTGATCCTGGCTCAGATTGAACGCTAGCGGGATGCCTTACACATGCAAGTCGAA
[0039] CGGCAGCACGGACTTCGGTCTGGTGGCGAGTGGCGAACGGGTGAGTAATGTATCGGAA
[0040] CGTGCCTAGTAGCGGGGGATAACTACGCGAAAGCGTAGCTAATACCGCATACGCCCTAC
[0041] GGGGGAAAGCAGGGGATCGCAAGACCTTGCACTATTAGAGCGGCCGATATCGGATTAGC
[0042] TAGTTGGTGGGGTAACGGCTCACCAAGGCGACGATCCGTAGCTGGTTTGAGAGGACGA
[0043] CCAGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAA
[0044] TTTTGGACAATGGGGGAAACCCTGATCCAGCCATCCCGCGTGTGCGATGAAGGCCTTCG
[0045] GGTTGTAAAGCACTTTTGGCAGGAAAGAAACGTCATGGGTTAATACCCCGTGAAACTGA
[0046] CGGTACCTGCAGAATAAGCACCGGCTAACTACGTGCCAGCAGCCGCGGTAATAC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com