Method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis
A microorganism and chlorophenyl technology, applied in the field of biocatalysis, can solve the problems of unfavorable pharmaceutical industry application, complicated operation process, unsatisfactory yield, etc., and achieve the effects of product safety, high enantioselectivity and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]Example 1 Screening of microbial strains with asymmetric reduction of 4-chlorophenyl-(pyridin-2-yl)-methanone
[0027] The experimental strains come from 4 soil samples of Changguangxi Wetland Park in Wuxi, Jiangsu Province. By using enrichment medium and screening plate medium, several microorganisms with certain tolerance to the substrate can be isolated. The substrate (4-chlorophenyl)-(pyridin-2-yl)-methanone and other reagents used in the experiment are commercially available analytically pure, chemically pure or biochemical reagents.
[0028] The main media used in the experiment include:
[0029] Enrichment medium (I): Glucose 50, yeast extract 20, potassium dihydrogen phosphate 4, magnesium sulfate heptahydrate 1.5, adjust pH to 6.0; make substrate into 500 g / L tetrahydrofuran solution, add 1 mL of substrate solution.
[0030] Screening plate medium (MSM) (Ⅱ): 0.2 ammonium sulfate, 0.2 potassium dihydrogen phosphate, 0.1 sodium chloride, 0.02 magnesium sulfate h...
Embodiment 2
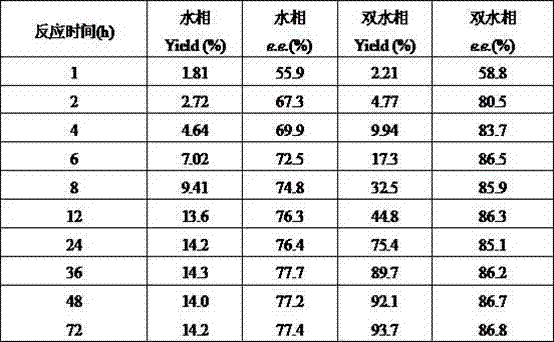
[0036] Influence of pH on CPMK asymmetric reduction reaction in the aqueous phase reaction system of embodiment 2
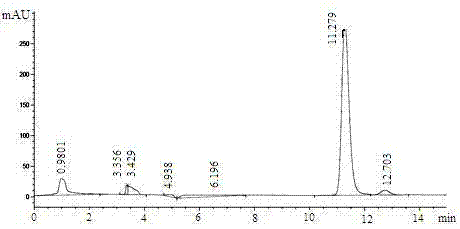
[0037] Suspend 1 g of wet cells in different buffer solutions containing 5% (W / V) glucose and 2 g / L CPMK (including: 0.2 M sodium acetate buffer (pH 4.5-5), potassium phosphate buffer (pH 6.0-7.5), Tris-HCl buffer (pH 8.0-8.5)), and set the volume to 10 mL, react at 30°C for 36 h, perform HPLC detection and calculate the reaction e.e. The values and yields are shown in Table 2.
[0038] pH Yield (%) e.e. (%) 4.5 12.5 78.9 5.0 12.5 72.6 5.5 17.0 71.1 6.0 68.1 70.9 6.5 76.9 70.9 7.0 81.3 74.6 7.5 79.7 71.4 8.0 76.7 66.1 8.5 47.2 66.4
[0039] It can be seen from Table 2 that the pH value of the reaction system has a significant impact on the activity of carbonyl reductase in the cells, and the cells maintain a high activity in the pH range of 6.0–8.0. The highest yield and e.e. values, ...
Embodiment 3
[0040] Embodiment 3 The influence of conversion reaction temperature on CPMK asymmetric reduction reaction
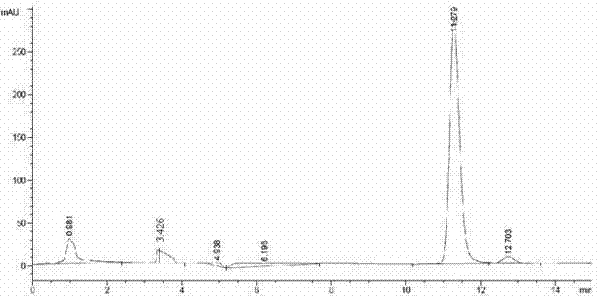
[0041] Suspend 1 g of wet cells in 0.2 M PBS (pH 7.0) containing 5% (W / V) glucose, 2 g / L CPMK and dilute to 10 mL. , 40 ℃, 45 ℃ for 36 h after the HPLC detection and calculation of the reaction e.e. The values and yields are shown in Table 3.
[0042] temperature(℃) Yield (%) e.e. (%) 25 75.4 78.2 30 79.2 78.2 35 3.79 -13.2 40 2.34 -22.4 45 0.923 -22.1
[0043] It can be seen from Table 3 that in the temperature range of 25 °C to 30 °C, e.e. Values (78.2%-78.3%) and yields (75.4%-79.2%) are relatively stable, and when the temperature rises to 35 ℃, e.e. value . and the yield dropped sharply to -13.2% and 3.79%, respectively, which indicated that the reaction temperature should be around 30 ℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com