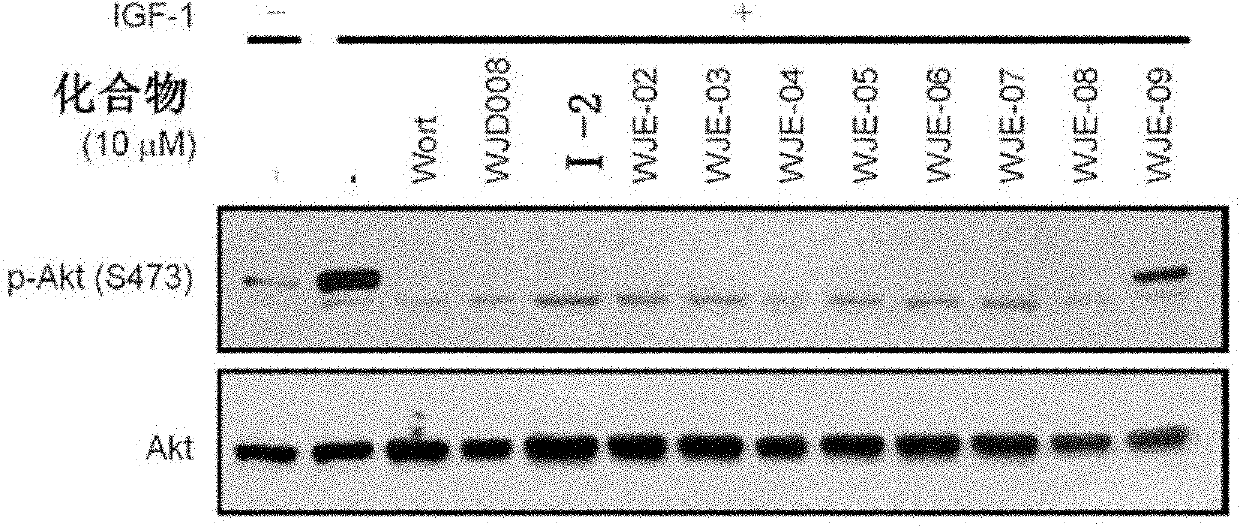
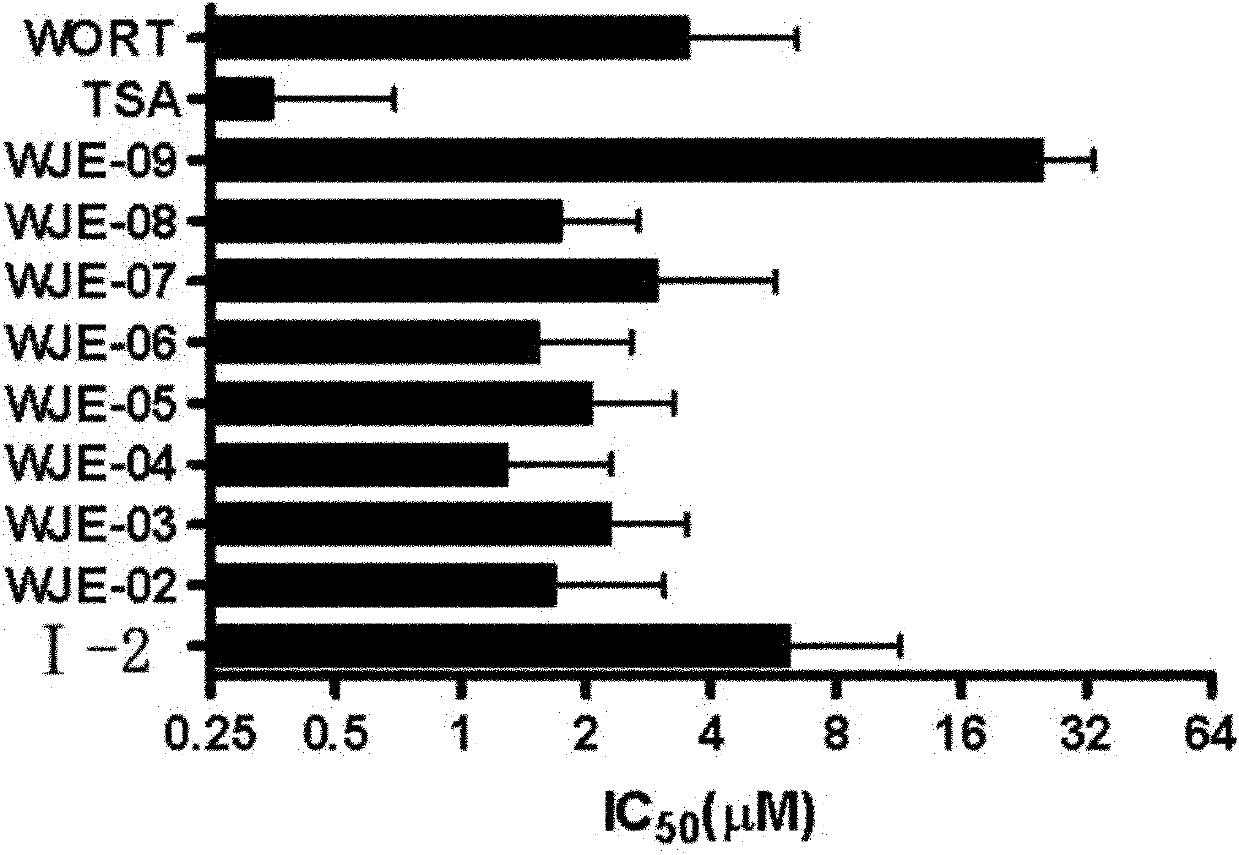
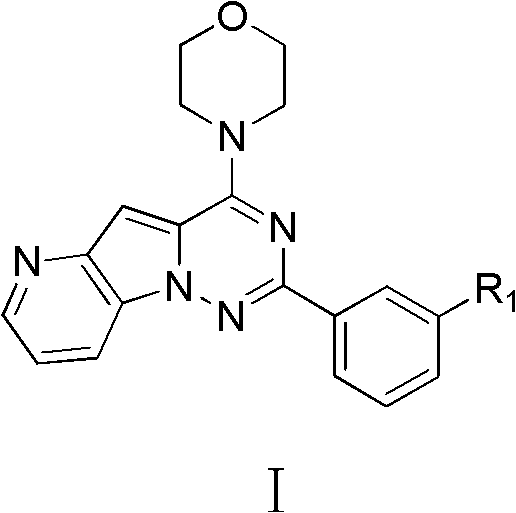
Pyrido-pyrrole triazine compound, and preparation method and application thereof
A technology of pyrroletriazine and compound, which is applied in the fields of pyrroletriazine compound, preparation and use thereof, and achieves the effects of enhanced activity and strong anti-proliferative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of the compound of the general formula I comprises the following steps:
[0026] a. Reaction of ethyl 4-azaindole-2-carboxylate (A) with freshly prepared chloramine under the action of a base to obtain compound B; the base is NaH or potassium tert-butoxide;
[0027] b. Combine B with R at the 3-position of the benzene ring 1 The substituted benzimidate hydrochloride is refluxed in ethanol to obtain compound C, wherein, in compound C, R 1 C is H, straight chain or branched chain 1 ~C 12 alkoxy or halogen;
[0028] c. Reacting C in phosphorus oxychloride at 80-120°C to obtain the chlorinated product D;
[0029] d, D is reacted with morpholine to obtain compound I-1, wherein, in compound I-1, R 1 C is H, straight chain or branched chain 1 ~C 12 alkoxy or halogen;
[0030] e. When R 1 C is a straight or branched chain 1 ~C 12 The compound I-1 in the case of alkoxy is dealkylated with boron tribromide or aluminum trichloride / ethanethiol to o...
Embodiment 1
[0041] 1, the preparation of 2-methyl-3-nitropyridine
[0042]
[0043] Add 479mg (20.8mmol) of sodium in batches to 8.6mL (56.8mmol, 9g) of diethyl malonate, stir for 15min, then add 3g (18.9mmol) of 2-chloro-3-nitropyridine in batches, 90 ℃ reaction 7h. Most of the diethyl malonate was distilled off under reduced pressure, then 20 mL of 36% sulfuric acid (weight percent) was added, and the reaction was carried out at 105° C. for 7 h. After the reaction is complete, wash with ether (50mL×2) and ethyl acetate (50mL×2) successively, discard the organic layer, adjust the pH of the aqueous layer to 8-9 with 1N sodium hydroxide solution, and extract with ethyl acetate (50mL×2). 3), dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure and separated on a silica gel column (petroleum ether / ethyl acetate=5 / 1) to obtain 2.1 g of a light yellow solid (yield: 80.4%). m.p.28~30℃.
[0044] 2, Preparation of 2-hydroxy-3-(3-nitropyridin-2-yl) ethyl acrylate...
Embodiment 2
[0074] 3-(4-Morpholinylpyrido[3',2':3,4]pyrrolo[1,2-f][1,2,4]triazazin-2-yl)phenyl acetate (WJE02 ) preparation
[0075]
[0076] Add 97mg (0.28mmol) of compound I-2, 0.040mL (0.43mmol, 43mg) of triethylamine and 3.5mg (0.028mmol) of DMAP into 4mL of anhydrous dichloromethane, slowly add 0.040mL (0.43mmol) , 43mg) acetic anhydride, react at room temperature. After the reaction was completed, it was washed with saturated brine (3×10 mL), dried over anhydrous sodium sulfate, and spin-dried in dichloromethane to obtain 93 mg of a yellow solid (yield: 85.2%). m.p.147-149°C.
[0077] 1 H NMR (300MHz, DMSO-d 6 )δ8.58(dd, J=4.3, 1.2Hz, 1H), 8.12(d, J=8.0Hz, 1H), 8.02(d, J=7.8Hz, 1H), 7.98-7.90(m, 1H), 7.87(t, J=7.9Hz, 1H), 7.64(s, 1H), 7.44(dd, J=8.4, 4.3Hz, 1H), 7.26(dd, J=8.3, 2.1Hz, 1H), 4.25-4.16 (t, J=4.9Hz, 4H), 3.88-3.80(t, J=4.9Hz, 4H), 2.28(s, 3H). MS (EI): m / e (%) 389 (100, M + ), 347(40).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com