Evodiamine prodrug containing indolequinone unit, and preparation method and application thereof
A technology of evodiamine and hydroxyevodiamine, which can be used in organic chemistry, drug combination, antitumor drugs, etc., and can solve problems such as lack of specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0034] 5-methoxy-2-methyl-1H-indole-3-aminomethyde (2). POCL will be used under stirring 3 (0.85 ml, 9.28 mmol) was added to DMF (N, N-dimethylformamide, 3.0 mL, 38.9 mmol) cooled to 0 ° C. After the addition, the Vilsmeier reagent was continued at 0 ° C for 15 minutes. 5-methoxy-2-methylindole (1.04 g, 6.45 mmol) was dissolved in 3 ml no water DMF, and cooled to 0 ° C, and previously prepared Vilsmeier reagents were added. After the addition was completed, the reaction was stirred at 0 ° C for 30 minutes, TLC point plate detection, after the reaction was completed, the reaction liquid was added to 2 m NaOH (50 mL) aqueous solution of 0 ° C, add DCM (dichloromethane, 100 mL) and Extraction separation. The aqueous layer was extracted again with DCM (50 mL). Combined with organic layers, washed with saturated brine, and use NA 2 SO 4 dry. The solvent was removed, and the residue was washed with a cooled EtOAc to give 1.05 g (86%) of Compound 2, which is a light brown solid. 1 H NMR ...
Embodiment 1
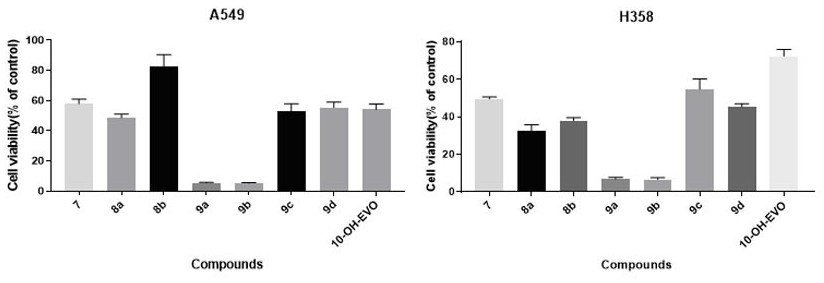
[0046] The synthesis of Compound 8a. Dikaric anhydride (51 mg, 0.51 mmol) was dissolved in DCM (2 mL), and DMAP (41.5 mg, 0.34 mmol) was added as a catalyst, and after stirring for 30 minutes, Compound 7 (40 mg, 0.17 mmol) was added, continued at room temperature. Stir 12 hours. Then washed with 10% by weight of hydrochloric acid (20 mL) and extracted with DCM (20 mL × 3). The combined organic layer was washed with saturated brine (20 mL) and used NA 2 SO 4 dry. After removing the solvent, the column chromatography was purified, eluted with EtOAc / petroleum ether (1: 1, RF = 0.21) to give a compound 8a (yield 73%) in the form of a red solid.
[0047] Synthesis of Compound 8b. Pentanic anhydride (58.2 mg, 0.51 mmol) was dissolved in DCM (2 mL), and DMAP (41.5 mg, 0.34 mmol) was added as a catalyst. After conventional stirring for 30 minutes, Compound 7 (40 mg, 0.17 mmol) was added, at room temperature. Stirring is stirred for 12 hours. Then washed with 10% by weight of hydrochlori...
Embodiment 2
[0056] The chemical structural formula of 10-OH-EVO is as follows:
[0057]
[0058] The synthesis of Compound 9a. 10-OH-EVO (15.95 mg, 0.05 mmol) was dissolved in DCM (2 mL), and DMAP (6.1 mg, 0.05 mmol) was added as a catalyst to the mixture. After completion, stirred at 0 ° C, adding compound 8a (12 mg, 0.05 mmol) and EDCI (0.25 mmol), and stirred at room temperature for 24 hours. The reaction mixture was then washed with 10% hydrochloric acid (20 mL) and extracted with DCM (20 mL × 3). The combined organic layers were washed with saturated sodium hydrogencarbonate (20 mL) and saturated brine (20 mL) and used NA 2 SO 4 dry. After removing the solvent, the residue was purified on silica gel, using DCM / CH 3 OH (4%, RF = 0.32) was eluted to afford the compound 9a (58% yield), which is a light red brown solid.
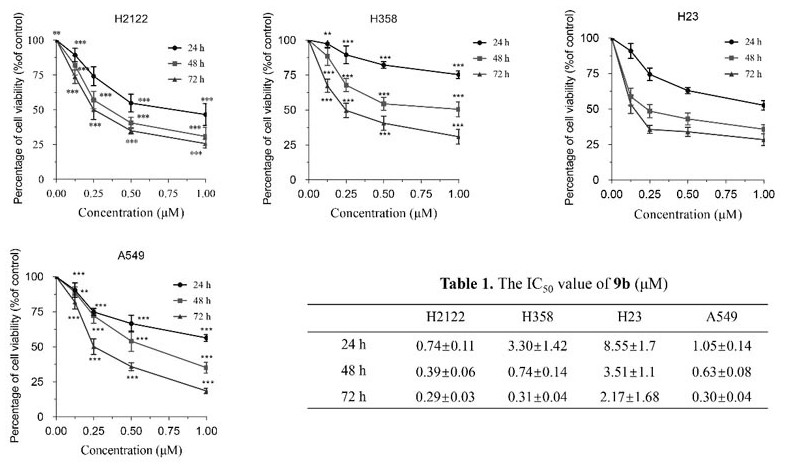
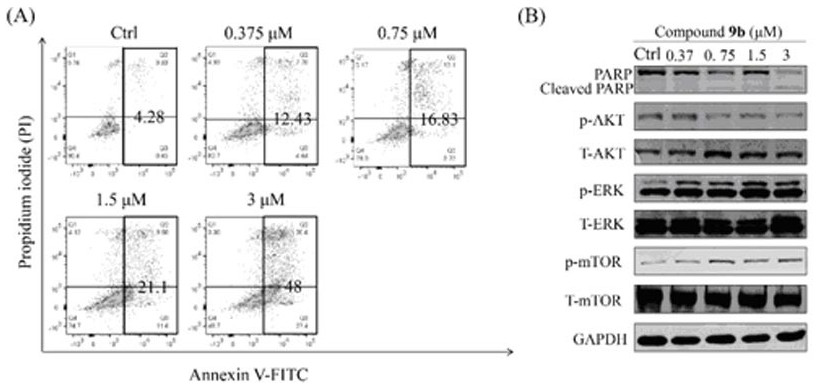
[0059]Synthesis of Compound 9b. 10-OH-EVO (15.95 mg, 0.05 mmol) was dissolved in DCM (2 mL), and DMAP (6.1 mg, 0.05 mmol) was added as a catalyst to the mixture. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com