Application of tanshinone I to treatment of microglia-mediated disease
A technology of microglia and tanshinone, applied in the field of tanshinone I, can solve the problems of decreased curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Effect of Tanshinone I on Microglia Activation-Induced Cell Death (AICD)
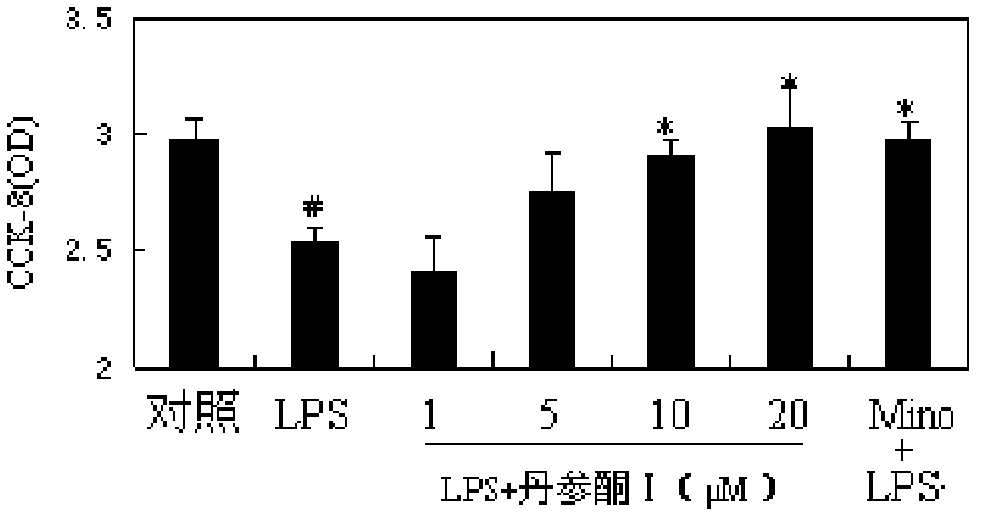
[0065] BV-2 cells were pre-incubated with tanshinone I (1, 5, 10, 20 μmol / L) for 0.5 h, and then stimulated with LPS (lipopolysaccharide, 0.1 μg / mL). After culturing for 24 hours, discard the supernatant, add CCK-8 (cell counting reagent, Guangzhou Yanchuang Biotechnology Co., Ltd.), continue to incubate for 30 minutes, and measure the absorbance at a wavelength of 450 nm. The experiment also set up the control group (normally growing cells), the model group (LPS) and the positive control group "Mino+LPS" group (5 μmol / L minocycline+lipopolysaccharide) in parallel. see results figure 1 .
[0066] The results showed that LPS can cause activation-induced cell death and significantly reduce the viability of BV-2 cells. Tanshinone I can inhibit LPS-induced activation-induced cell death in a dose-dependent manner.
[0067] In addition, "extract A" and "extract B" were tested as reagen...
Embodiment 2
[0068] Example 2: Effect of Tanshinone I on Viability of Microglial Cells
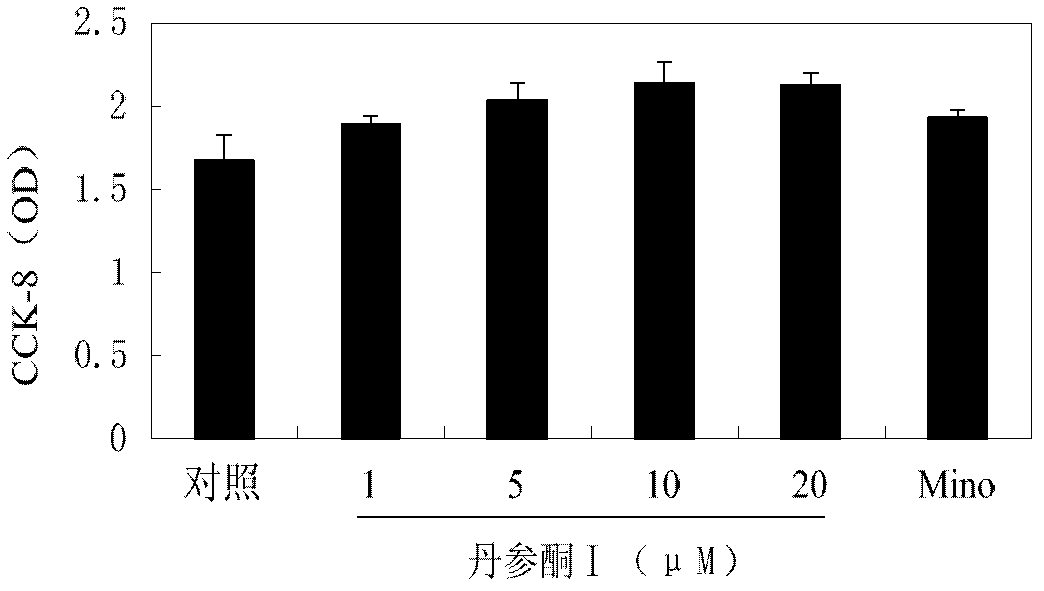
[0069] After BV-2 cells were cultured with tanshinone I (1, 5, 10, 20 μmol / L) for 24 hours, discard the supernatant, add CCK-8, continue to incubate for 30 minutes, and measure the absorbance at a wavelength of 450 nm. In the experiment, the control group (cells with normal growth) and the "Mino" group were set up in parallel. see results figure 2 .
[0070] The results showed that tanshinone I could not inhibit cell viability and did not cause cell death.
Embodiment 3
[0071] Example 3: Effect of Tanshinone I on Secretion of Inflammatory Mediators by Microglia
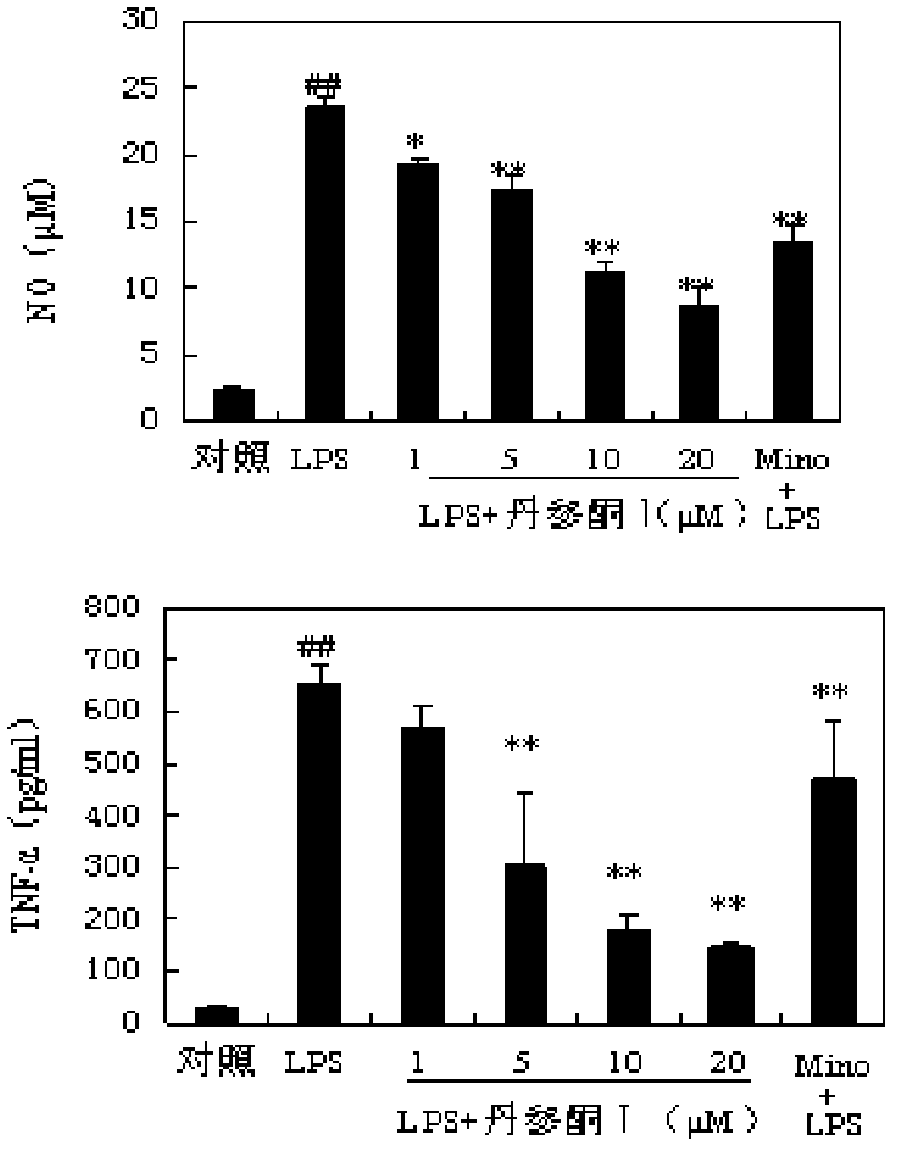
[0072] The mouse microglial cell line BV-2 was cultured in complete DMEM medium. Inoculate into 48-well culture plate overnight, add different concentrations of Tanshinone I (final concentration 1, 5, 10, 20 μmol / L) for pre-incubation for 0.5 h, and then add endotoxin (LPS, final concentration 0.1 μg / mL) for stimulation . Experimental groups: normal control group, LPS group, Tanshinone I plus LPS group, and "Mino+LPS" group. The concentration of NO was detected by Greiss method, and the concentration of TNF-α was measured by Elisa kit. see results image 3 .
[0073] The results showed that Tanshinone I could significantly inhibit the release of NO and TNF-α induced by LPS in a dose-dependent manner within the concentration range of 1-20 μmol / L.
[0074] In addition, "extract A" and "extract B" were tested as reagents, and the two extracts showed the same dose as the pure tans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com