Orally disintegrating tablet of 5-HT receptor agonist and preparation method thereof
An oral disintegrating tablet and receptor agonist technology, which is applied in the directions of pill delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
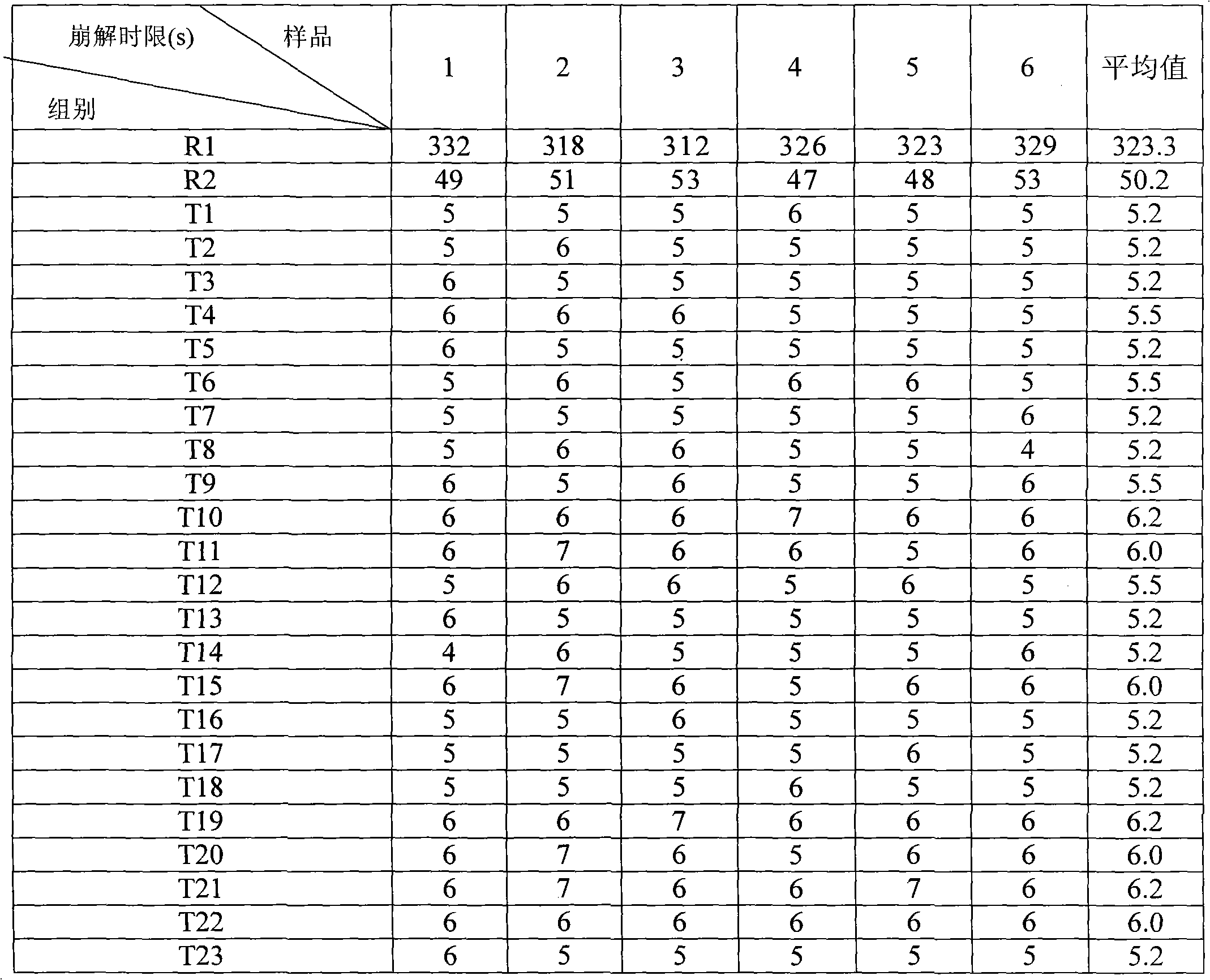
Image
Examples
Embodiment 1
[0152] The preparation formula of the present invention is made up of following components:
[0153] Zolmitriptan 2.50g
[0154] Mannitol 7.00g
[0155] Pullulan 9.00g
[0156] Xanthan gum 0.06g
[0157] Acesulfame K 0.50g
[0158] Mint essence 0.20g
[0159] Purified water 180.74g
[0160] A total of 1000 pieces were made.
[0161] The specific preparation method is as follows: Zolmitriptan, mannitol, pullulan, acesulfame potassium, and peppermint essence are added to the fully dissolved xanthan gum solution, and mixed to form a homogeneous solution; After degassing, pour it into the mold accurately; pre-freeze at -40°C to -170°C for 1 to 60 minutes, then transfer it to a freeze dryer, under the conditions of 0.01mbar to 10mbar and -30°C to 30°C Freeze-dry for 1-10 hours to obtain zolmitriptan orally disintegrating tablets of the present invention; in the above method, after the pre-freezing step, before transferring to the lyophilizer, an ice crystal incubation step c...
Embodiment 2
[0163] The preparation formula of the present invention is made up of following components:
[0164] Zolmitriptan 2.50g
[0165] Glycine 7.00g
[0166] Pullulan 9.00g
[0167] Xanthan gum 0.06g
[0168] Acesulfame K 0.50g
[0169] Mint essence 0.20g
[0170] Purified water 180.74g
[0171] A total of 1000 pieces were made.
[0172] The specific preparation method is as follows: add zolmitriptan, glycine, pullulan, acesulfame potassium, and peppermint essence into the fully dissolved xanthan gum solution, and mix to form a homogeneous solution; the following preparation method is the same as Example 1.
Embodiment 3
[0174] The preparation formula of the present invention is made up of following components:
[0175] Zolmitriptan 2.50g
[0176] Glycine 3.20g
[0177] Mannitol 3.80g
[0178] Sodium alginate 7.00g
[0179] Konjac gum 0.36g
[0180] Sucralose 0.16g
[0181] Mint essence 0.20g
[0182] Purified water 182.78g
[0183] A total of 1000 pieces were made.
[0184] The specific preparation method is as follows: Zolmitriptan, glycine, mannitol, sodium alginate, sucralose, and peppermint flavor are added to the fully dissolved konjac gum solution, and mixed to form a uniform solution; the following preparation Method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com