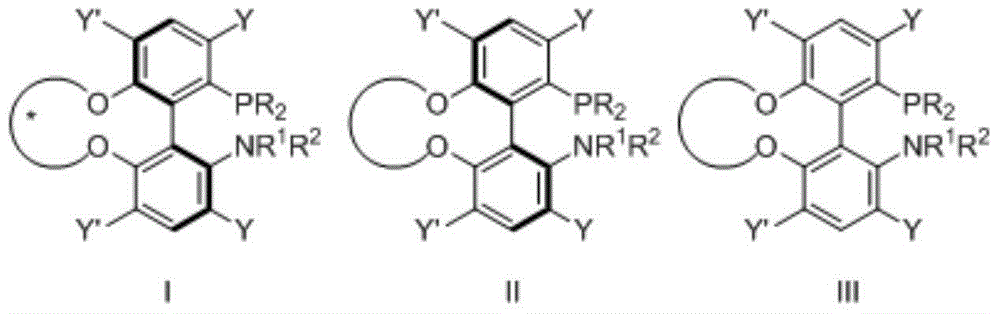
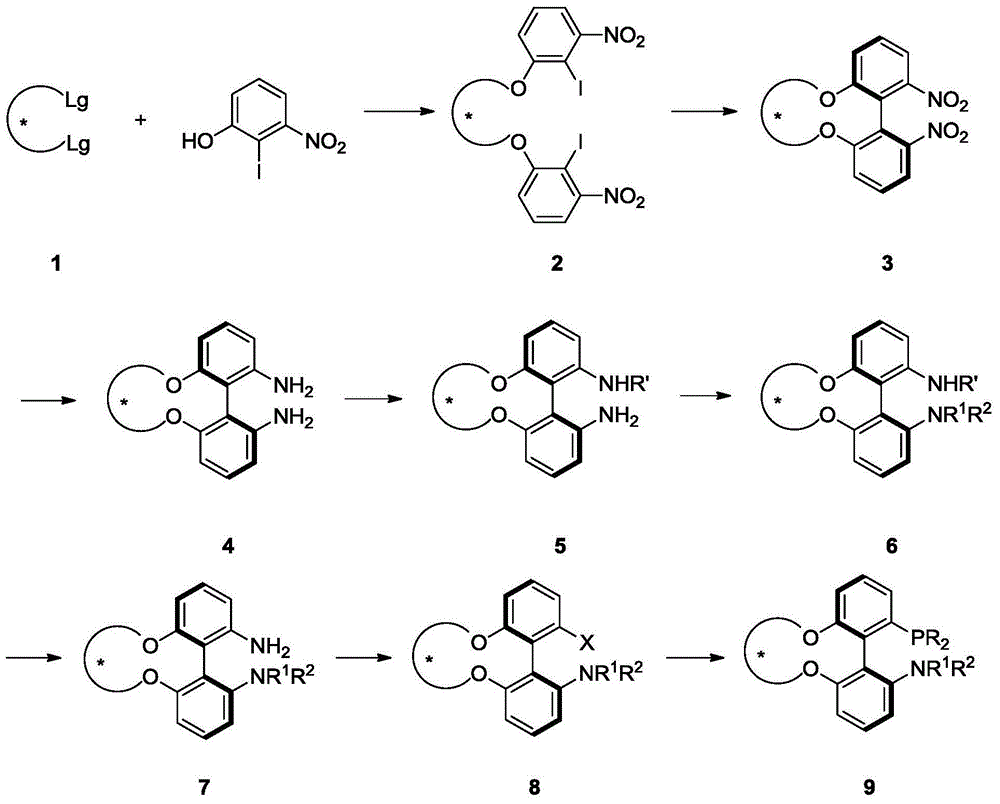
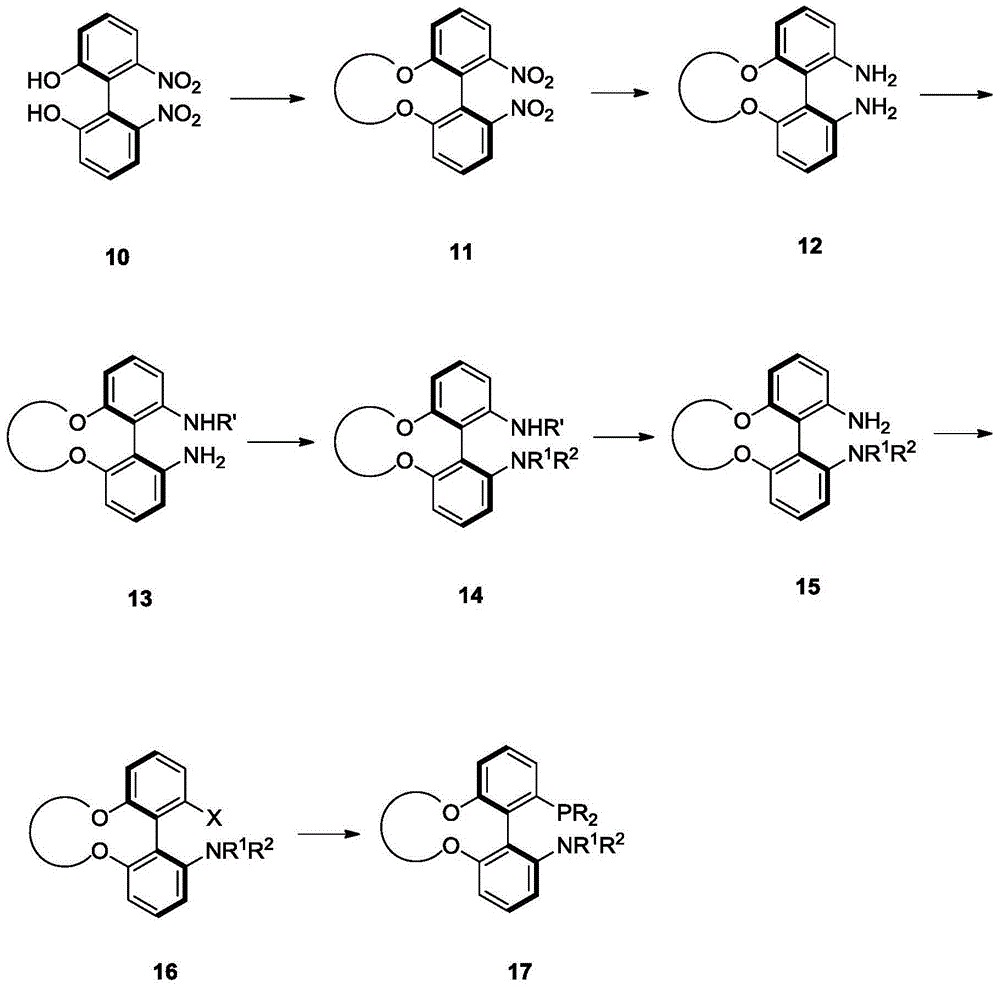
Phosphine ligand and its enantiomer or racemate and its preparation method
A technology of enantiomers and phosphine ligands, which is applied in the field of phosphine ligands or their enantiomers or their racemates and their preparation, and can solve the problems of insufficient number and types of ligands, single skeleton space structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: To prepare (S)-[6,6'-((2R,5R)-2,5-hexanedioloxy)]-2-N,N-dimethylamino-2'-bicyclic Hexylphosphine-1,1'-biphenyl as an example
[0058]
[0059] Preparation of (2R,5R)-2,5-bis-[(2-iodo-3-nitro)phenoxy]hexane:
[0060] 2-iodo-3-nitrophenol (5.30g, 0.02mol) and potassium carbonate (5.60g, 0.04mol) were added to 60mL of N,N-dimethylformamide (DMF), and stirred down to the mixing system Add (2S,5S)-2,5-hexanediol bis-p-toluenesulfonate (4.27g, 0.01mol), heat at 55°C until the reaction is complete (TLC tracking), and distill off DMF under reduced pressure. residues in water and CH 2 Cl 2 Partition in the middle, separate the organic phase and use CH for the aqueous phase 2 Cl 2 The organic phases were extracted and combined, washed with saturated brine, dried over anhydrous magnesium sulfate, the solvent was removed under reduced pressure, and purified by column chromatography to obtain 5.10 g of a yellow solid compound with a yield of 83%. 1 H NMR (CDCl 3...
Embodiment 2
[0075] Example 2: To prepare (R)-[6,6'-((2S,3S)-2,3-butanediol oxygen)]-2-N,N-dimethylamino-2'-bicyclic Hexylphosphine-1,1'-biphenyl as an example
[0076]
[0077] Preparation of (2S,3S)-2,3-bis-[(2-iodo-3-nitro)phenoxy]butane:
[0078] Add 2-iodo-3-nitrophenol (5.30g, 0.02mol) and potassium carbonate (5.60g, 0.04mol) to 60mL N, N-dimethylformamide (DMF), and add to the mixing system under stirring (2R,3R)-2,3-Butanediol bis-p-toluenesulfonate (3.98g, 0.01mol), heated at 80°C until the reaction was complete (TLC tracking), and distilled off DMF under reduced pressure, the residue in water and CH 2 Cl 2 Partition in the middle, separate the organic phase and use CH for the aqueous phase 2 Cl 2 Extracted, combined organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, removed the solvent under reduced pressure, and purified by column chromatography to obtain 3.68 g of a yellow solid compound with a yield of 63%. 1 H NMR (CDCl 3 , TMS, 300...
Embodiment 3
[0093] Example 3: (S)-[6,6'-((2R,5R)-2,5-hexanedioloxy)]-2-N,N-dimethylamino-2'-dicyclohexylphosphine - Application of 1,1'-biphenyl in the asymmetric Suzuki reaction.
[0094]
[0095] In the glove box, 2-diethylphosphite-1-bromonaphthalene, (1.0mmol, 1.0equiv), 2-methylboronic acid (1.5equiv), Pd 2 (dba) 3 (0.5mol%), ligand (S)-[6,6'-((2R,5R)-2,5-hexanedioloxy)]-2-N,N-dimethylamino-2'- Dicyclohexylphosphine-1,1'-biphenyl (1.2 mol%) and potassium phosphate (3 equiv) were placed in a 6 mL dry one-necked bottle, the bottle was closed, and stirred at room temperature for 48 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com