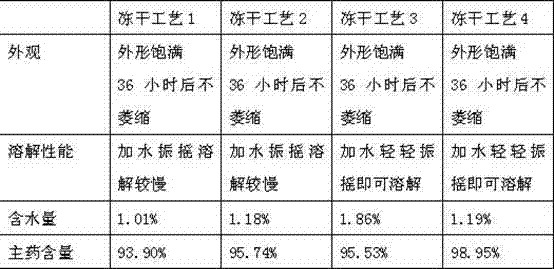
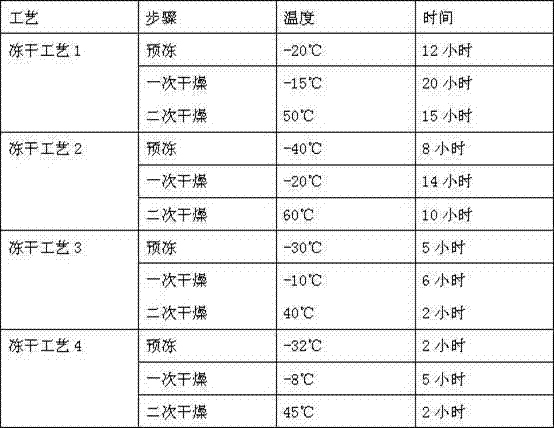
Lyophilized emulsion preparation for injection of levo-gossypol and acetate of levo-gossypol
A technology of L-gossypol and acetate, which is applied in the direction of freeze-drying transportation, emulsion transportation, active ingredients of aldehydes, etc. It can solve the problems of poor stability and achieve the effect of strong stability, low moisture content and full appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of lyophilized milk preparation for L-gossypol injection
[0035] The formula is as follows:
[0036] L-Gossypol 10mg
[0037] Oleic acid 980mg
[0038] Glycerin 4.8g
[0039] Soy Lecithin 31.2mg
[0040] Span 80 7.8mg
[0041] Trehalose 14mg
[0042] Human Albumin 14mg
[0043] Sodium sulfite 0.21mg
[0044] Soybean oil for injection 17.2g
[0045] Water for injection 10L
[0046] The preparation method is as follows:
[0047] 1) mixing L-gossypol, soybean oil for injection, soybean lecithin, Span 80, and sodium sulfite to make an oil phase;
[0048] 2) Mix water for injection, glycerin and oleic acid, add sodium hydroxide to adjust the pH to 9.0-10.5;
[0049] 3) Pour the oil phase into the water phase to prepare colostrum;
[0050] 4) Use a high-pressure homogenizer to homogenize the colostrum to make final milk;
[0051] 5) Add treha...
Embodiment 2
[0056] Example 2 Preparation of lyophilized milk preparation for L-gossypol acetate injection
[0057] The formula is as follows:
[0058] L-Gossypol Acetate 10mg
[0059] Oleic acid 1200mg
[0060] Glycerin 4.5g
[0061] Soy Lecithin 27.2mg
[0062] Span 80 6.8mg
[0063] Trehalose 9.5mg
[0064] Human Albumin 9.5mg
[0065] Sodium sulfite 0.26mg
[0066] Soybean oil for injection 19.7g
[0067] Water for injection 10L
[0068] The preparation method is as follows:
[0069] 1) mixing L-gossypol acetate, soybean oil for injection, soybean lecithin, Span 80, and sodium sulfite to make an oil phase;
[0070] 2) Mix water for injection, glycerin and oleic acid, add sodium hydroxide to adjust the pH to 9.0-10.5;
[0071] 3) Pour the oil phase into the water phase to prepare colostrum;
[0072] 4) Use a high-pressure homogenizer to homogenize the colostrum to make final...
Embodiment 3
[0078] Example 3 Preparation of lyophilized milk preparation for L-gossypol injection
[0079] The formula is as follows:
[0080] L-Gossypol 10mg
[0081] Oleic acid 1300mg
[0082] Glycerin 4.2g
[0083] Soy Lecithin 25.6mg
[0084] Span 80 6.4mg
[0085] Trehalose 12mg
[0086] Human Albumin 12mg
[0087] Sodium sulfite 0.22mg
[0088] Soybean oil for injection 14.1g
[0089] Water for injection 10L
[0090] The preparation method is as follows:
[0091] 1) mixing L-gossypol, soybean oil for injection, soybean lecithin, Span 80, and sodium sulfite to make an oil phase;
[0092] 2) Mix water for injection, glycerin and oleic acid, add sodium hydroxide to adjust the pH to 9.0-10.5;
[0093] 3) Pour the oil phase into the water phase to prepare colostrum;
[0094] 4) Use a high-pressure homogenizer to homogenize the colostrum to make final milk;
[0095] 5) Add tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com