Dibenzyl-macrolide compound based on photoreaction and synthesis method thereof
A biaryl compound technology, applied in the field of chemical synthesis, can solve problems such as synthesis difficulties, and achieve the effects of simple operation, wide and easy-to-obtain raw material sources, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
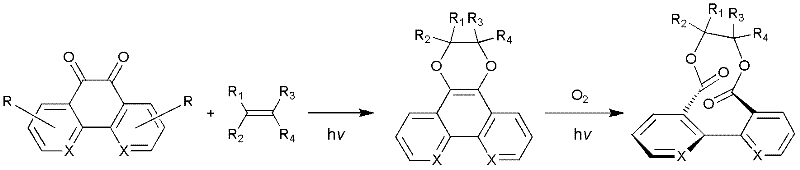
[0039] Phenanthrene-9,10-dione (PQ) and 2 times the molar amount of olefin are dissolved in benzene (PQ concentration is 0.02M). Under light (400nm-760nm) conditions, the reaction was detected by TLC. After the PQ was consumed, the solvent was evaporated under reduced pressure, and the same volume of anhydrous acetonitrile was added to dissolve it. Then oxygen was added to the reaction solution and continued to λ≥400nm- After the reaction is irradiated with light at 760nm, column chromatography can obtain the biphenyl ten-membered ring dilactone compound. The reaction equation is shown in the following formula.
[0040]
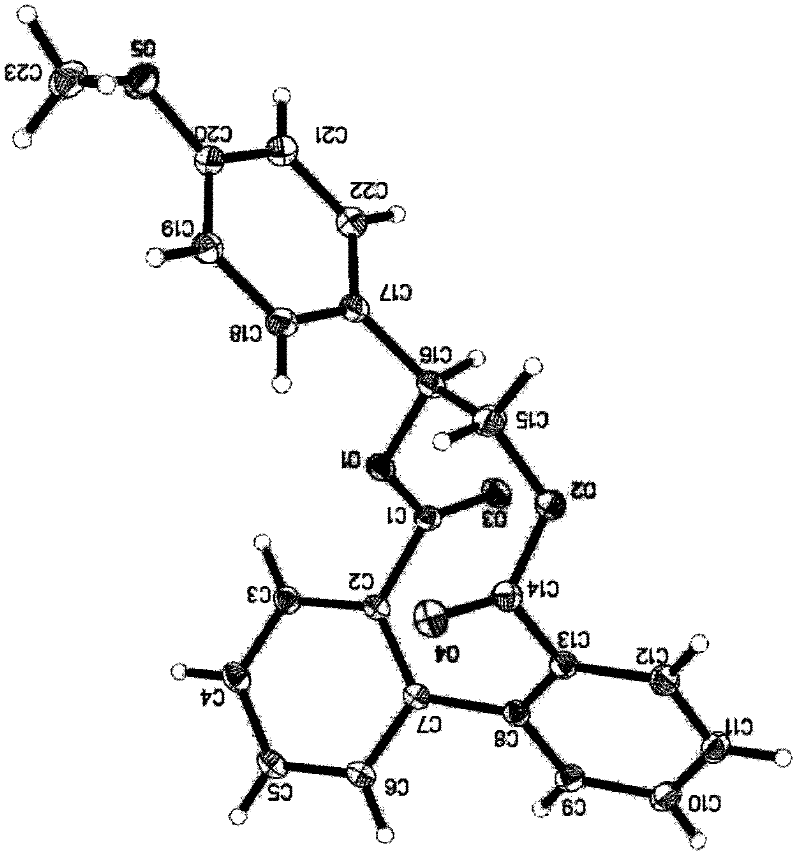
[0041] The olefin cyclopropylidene cyclopropane was used in Example 1 to obtain biphenyl ten-membered ring dilactone 3a, white powder; m.p.192-194℃; 1 H NMR(300MHz, CDCl 3 ): δ 7.58-7.36 (m, 8H), 1.78-1.69 (m, 2H), 1.44-1.35 (m, 2H), 0.99-0.90 (m, 2H), 0.28-0.19 (m, 2H) ppm; 13 C NMR(75MHz, CDCl 3 ): δ170.0(2C), 137.9(2C), 133.7(2C), 131.1(2C), 131.0(2C), 127.8(...
Embodiment 2
[0058] 1,10-phenanthroline-5,6-dione (PN) and 2 times the molar amount of olefin are dissolved in anhydrous acetonitrile (PN concentration is 0.02M). Reaction under light (400nm-760nm) conditions, the reaction is detected by TLC, after the PN is consumed, oxygen is supplied to the reaction solution and irradiated with λ≥300nm-760nm light. After the reaction is over, column chromatography can obtain the coupling For pyrido ten-membered ring dilactone compounds, the reaction equation is shown in the following formula.
[0059]
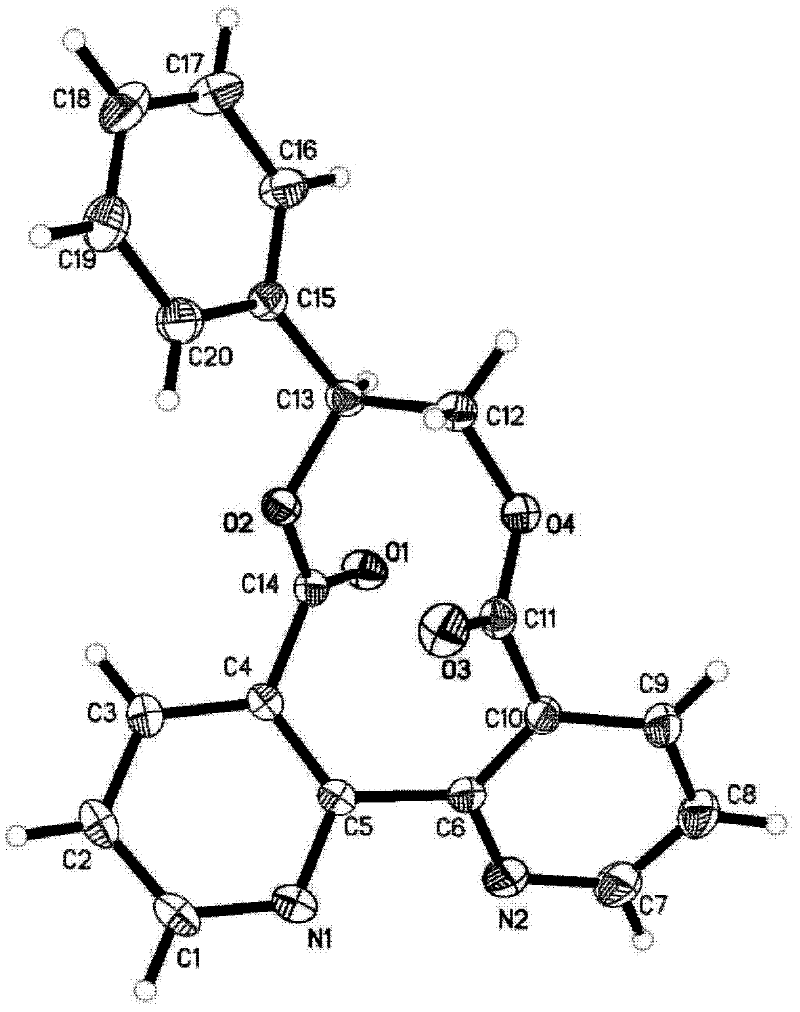
[0060] The olefin cyclopropylidene cyclopropane was used in Example 2 to obtain bipyrido ten-membered ring dilactone 5a, white powder; m.p.254-256℃; 1 H NMR(300MHz, CDCl 3 ): δ8.93 (d, J = 3.6 Hz, 2H), 7.83 (d, J = 7.5 Hz, 2H), 7.41 (dd, J = 7.5, 4.8 Hz, 2H), 1.78-1.65 (m, 2H) , 1.45-1.33 (m, 2H), 1.03-0.94 (m, 2H), 0.33-0.24 (m, 2H) ppm; 13 C NMR(75MHz, CDCl 3 ): δ168.6, 153.6, 152.0, 134.4, 130.3, 123.0, 64.5, 14.1, 6.7ppm; IR(KBr)v max / cm -1 : 1759, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com