Method for selectively oxidizing sulfide
A sulfide and selective technology, which is applied in the direction of chemical instruments and methods, preparation of organic compounds, chemical/physical processes, etc., can solve problems such as not suitable for large-scale application and high price, and achieve high selectivity, less three wastes, and High conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
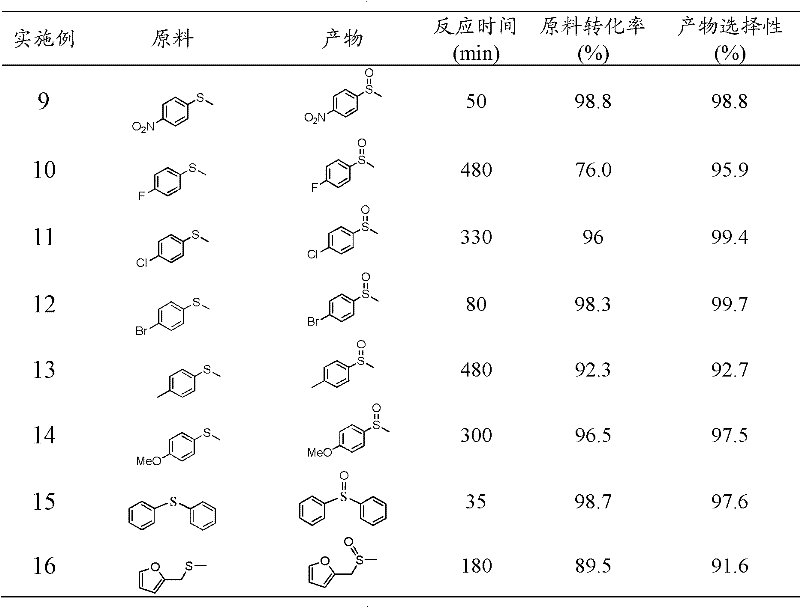
Examples
Embodiment 1
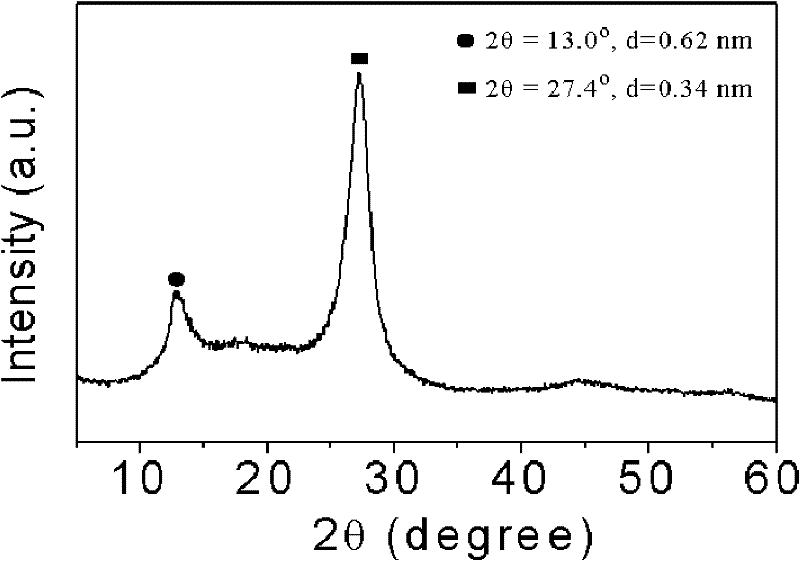
[0019] Take 3g of cyanamide, dissolve it in 3g of water, add 407.5g of Ludox HS, raise the temperature to 80°C to remove water, and obtain a white solid, heat up to 550°C at 2.5°C / min to polymerize for 4h, cool to room temperature and use 4M / L The ammonium bifluoride solution removes the template agent, and after drying, a yellow mesoporous carbon nitride material is obtained; XRD analysis has two peaks, and 2θ=27.4° is the (002) peak of the material, representing that the distance between the material layers is 0.34nm , 2θ=13.0° is the (100) peak of the material, which represents the regularity of the layered structure of the material, and the specific surface area of the material is 200m 2 / g.
[0020] Put 50mg of mesoporous carbon nitride catalyst into a 50ml three-neck round-bottomed flask, add 3ml of acetonitrile and 2mmol of isobutyraldehyde as a cocatalyst, take 1mmol of raw material sulfide anisole into the round-bottomed flask, and react with oxygen (bubbling) at 25...
Embodiment 2
[0022] Put 50 mg of the mesoporous carbon nitride catalyst prepared in Example 1 into a 50 ml three-necked round-bottomed flask, add 3 ml of acetonitrile, take 1 mmol of the raw material sulfide anisole and add it to the round-bottomed flask, and react with oxygen (bubbling), and react at 25 ° C for 8 Hour. After the reaction was completed, it was filtered and washed three times with ethanol. Gas chromatography analysis showed that the conversion rate of sulfide anisole was 27.6%, and the selectivity of sulfoxide was 98.9%.
Embodiment 3
[0024] Put 50mg of the mesoporous carbon nitride catalyst prepared in Example 1 into a 50ml three-necked round-bottomed flask, add 3ml of acetonitrile and 2mmol of benzaldehyde as a cocatalyst, take 1mmol of the raw material sulfide anisole and add it to the round-bottomed flask, and pass through oxygen (bubbling) The reaction was carried out at 25°C for 8 hours. After the reaction was completed, it was filtered and washed three times with ethanol. Gas chromatography analysis showed that the conversion rate of sulfide anisole was 35.2%, the selectivity of sulfoxide was 78.5%, and the selectivity of sulfone was 20.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com