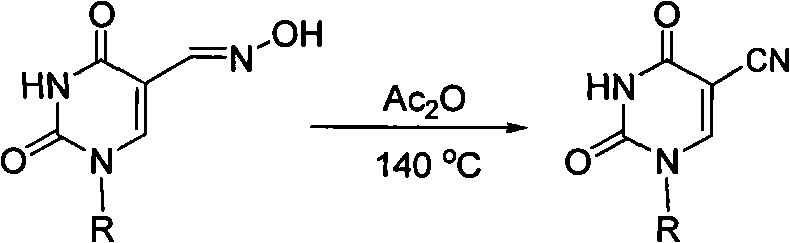
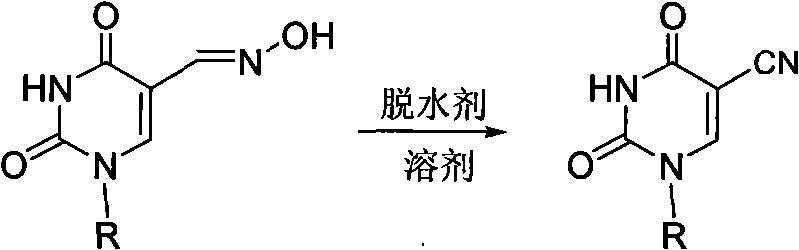
Method for synthesizing 5-cyano pyridine nucleoside derivatives
A technology of cyanopyrimidine nucleoside derivatives and pyrimidine nucleosides, which is applied in the field of preparation of 5-cyanopyrimidine nucleoside derivatives, can solve the problems of difficult recovery of solvents, harsh reaction conditions, expensive raw materials, etc., and achieve easy recovery and The effect of repeated use and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Synthesis of Example 1.5-cyano-2'-deoxy-3',5'-di-O-acetylated uridine
[0017] Acetic anhydride and 2'-deoxy-3',5'-di-O-acetyluridine-5-carbaldehyde oxime (355 mg, 1 mmol) were added to a 25 mL flask. Then, the reaction bottle was placed in an oil bath, and reacted at a temperature of 140° C. for 3 hours. After the reaction, concentrate under reduced pressure (recover acetic anhydride), add water, precipitate 5-cyano-2'-deoxy-3',5'-bis-O-acetylated uridine crude product, and filter with suction. The crude product was recrystallized from ethanol-water to obtain 296 mg (88%) of the product 5-cyano-2'-deoxy-3',5'-bis-O-acetylated uridine.
[0018] White solid.mp 161-162℃; 1 H NMR (DMSO-d 6 , 400MHz) δ: 2.04(s, 6H, 2CH 3 ), 2.36-2.39(m, 1H, CH), 2.51-2.55(m, 1H, CH), 4.22-4.25(m, 3H, CH, CH2), 5.18(s, 1H, CH), 6.06(t, J=6.4Hz, 1H, CH), 8.56(s, 1H, CH), 12.07(s, 1H, NH); 13 C NMR δ: 20.92, 21.10, 36.96, 63.81, 73.80, 82.32, 86.50, 89.15, 114.58, 149.35, 150.16, 160.37,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com