Synthesis method for guanoxan sulfate
A technology of guanidine sulfate production and synthesis method, applied in the field of compound synthesis, can solve the problems of low yield, complicated steps and the like, and achieve the effects of high yield, simple steps and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
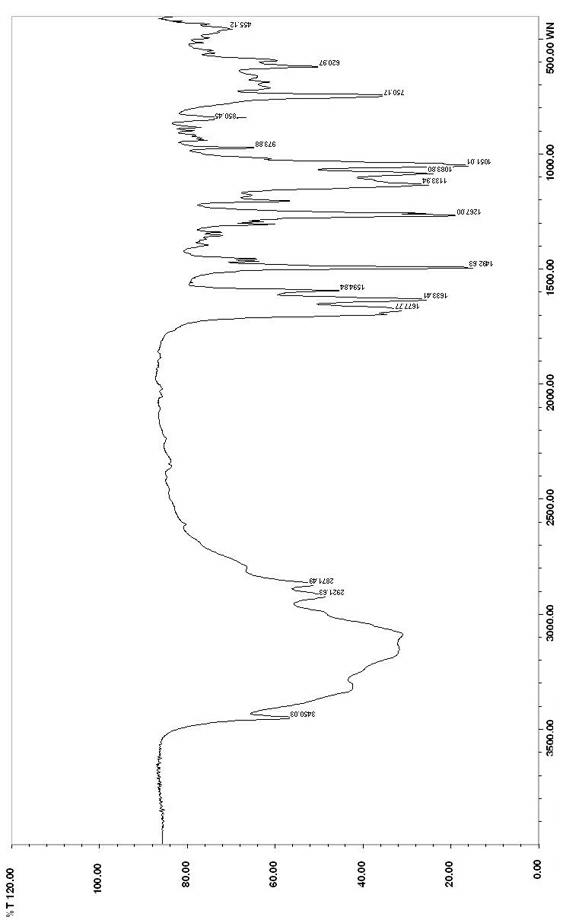
[0020] (1) 2-bromomethyl-1,4-benzodioxane
[0021] Add 50g of 2-hydroxymethyl-1,4-benzodioxane and 200ml of chloroform into the reactor, stir for 30 minutes to dissolve, and add 90g of phosphorus tribromide dropwise at a temperature not exceeding 30°C. After the dropwise addition is completed, Raise the temperature to 50°C and react for 4 hours. Stop the reaction when there is no raw material by TLC. After recovering chloroform under reduced pressure, collect the fractions at 110°C-145°C under the condition of vacuum degree of 0.095 to obtain the oily substance 2-bromomethyl-1,4 - Benzodioxane 55g, yield 79.7%;
[0022] (2) 1,4-Benzodioxane-2-methylamine
[0023] Add 55g of 2-bromomethyl-1,4-benzodioxane, 275ml of toluene, and 48.5g of potassium phthalimide to the reactor in sequence, heat up to 110°C and reflux for 3 hours, then add 85 % Hydrazine hydrate 28g continued to reflux at 110°C for 3 hours, TLC detected that the reaction was complete, cooled to 25°C and crystalliz...
Embodiment 2
[0027] (1) 2-Chloromethyl-1,4-benzodioxane
[0028] Add 45g of 2-hydroxymethyl-1,4-benzodioxane, 180ml of chloroform, and 40.5g of thionyl chloride into the reactor, stir for 30 minutes to dissolve, raise the temperature to 40°C and react for 4 hours, when no raw materials are detected by TLC After stopping the reaction and recovering chloroform, the fraction at 100°C-130°C was collected under a vacuum of -0.095 to obtain 42 g of oily 2-chloromethyl-1,4-benzodioxane, with a yield of 83.9%;
[0029] (2) 1,4-Benzodioxane-2-methylamine
[0030] Add 40g of 2-chloromethyl-1,4-benzodioxane, 200ml of benzene, and 44g of potassium phthalimide to the reactor in sequence, raise the temperature to 110°C for reflux reaction for 5 hours, and add 85% hydration Hydrazine 25.5g continued to reflux at 110°C for 4 hours, TLC detected that the reaction was complete, cooled to 25°C and crystallized for 8 hours, filtered and dried to obtain 31g of the product 1,4-benzodioxane-2-methylamine, yield...
Embodiment 3
[0034] (1) 2-bromomethyl-1,4-benzodioxane
[0035] Add 50g of 2-hydroxymethyl-1,4-benzodioxane and 750ml of toluene to the reactor, stir for 30 minutes, add 165g of phosphorus tribromide dropwise at a temperature not exceeding 40°C, after the dropwise addition, raise the temperature Reaction at 80°C for 1 hour, after the toluene was recovered under reduced pressure, the fraction at 110°C-145°C was collected under the condition of vacuum degree of 0.095 to obtain 57g of oily 2-bromomethyl-1,4-benzodioxane, which was collected The rate is 82.6%;
[0036] (2) 1,4-Benzodioxane-2-methylamine
[0037] Add 55g of 2-bromomethyl-1,4-benzodioxane, 660ml of toluene, and 97.9g of phthalimide potassium salt to the reactor in sequence, raise the temperature to 90°C for 5 hours, add 85% hydration Hydrazine 70.4g, continued to react at 90°C for 4 hours, TLC detected that the reaction was complete, cooled to 25°C and crystallized for 8 hours, filtered and dried to obtain 32g of the product 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com