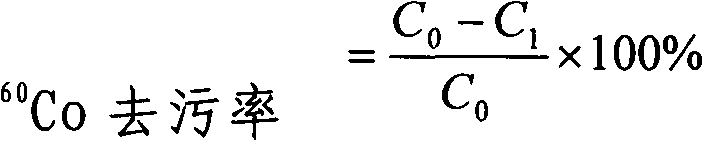Decontamination bag for skin radioactive contamination
A radioactive and decontamination technology, applied in the field of decontamination bags, to reduce wound infection, avoid irradiation, and achieve good decontamination effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1, decontamination effect experiment
[0016] 1. Selection and acquisition of radionuclides
[0017] Buy CoCl 2 , by low dose rate fission neutrons ( 252 Cf, absorbed dose rate 0.35mGy / h) irradiated for 2 months to produce activated products 60 Co, 131 I was provided by Huangpu District Central Hospital.
[0018] 2. Establishment of wound and abrasion models
[0019] Small fragrant pigs, 40mg / kg, intraperitoneal injection of pentobarbital sodium, skin preparation, skin well-shaped incision.
[0020] 3. Screening of physical materials
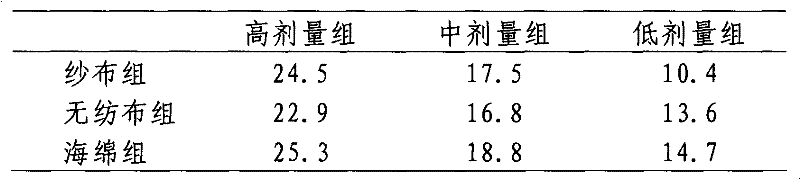
[0021] Select microporous sponges, non-woven fabrics, and medical gauze (materials with high adsorption capacity and gentle contact surface), respectively take materials of the same area and weight, and add physiological saline dropwise until it oozes out to compare water absorption; , skin preparation, cutting 3cm × 3cm, divided into 4 groups (blank group, sponge wiping group, non-woven cloth wiping group, medical gauze...
Embodiment 2
[0049] Embodiment 2, stimulating experiment
[0050] 1. Wound irritation test:
[0051] New Zealand rabbits, 8 rabbits, half male and half male, the hair on both sides of the back was cut short and then depilated. The depilated area was 3cm×3cm each. Group (intact skin group I, damaged skin group II), experimental group (intact skin group III, damaged skin group IV), artificial wounds, 0.5ml (g) of the mixed group decontamination solution was directly coated on the wounds, and used Cover and fix with two layers of gauze (2.5cm×2.5cm) and a layer of cellophane. The skin on the other side is used as a control. Apply the negative control and apply for 4 hours. After the test, remove the remaining test samples with warm water. Observe the skin reaction at the site where the test sample was administered 1, 24, 48, and 72 hours after the test sample was removed, and determine the wound irritation intensity. According to the standard score of skin erythema, edema formation and woun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com