Method for converting 3-cyanopyridine into nicotinic acid by using gibberella intermedia CA3-1
A technology of cyanopyridine and gibberella, which is applied in the biological field and can solve problems such as long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 Utilizes Gibberella CA3-1 to convert 3-cyanopyridine into nicotinic acid
[0017] (1) Preparation of cell liquid culture of Gibberella
[0018] Pick a ring of Gibberella strains on the solid Chapei culture medium, inoculate it in a 250mL Erlenmeyer flask containing 30mL seed medium, and culture it on a shaker at 200r / min at 30°C for 20-24h to Logarithmic phase, that is, the cell liquid culture of the strain.
[0019] (2) The composition and proportioning of the fermentation medium are:
[0020] Carbon source 50-100g / L; organic nitrogen source 10-30g / L; NH 4 Cl 20-30g / L; K 2 HPO 4 0.1-1.0g / L; MgSO 4 0.1-1g / L; CuCl 2 0.01-0.1g / L; ZnSO 4 0.01-0.10g / L; MnSO 4 0.01-0.10g / L; FeSO 4 ·7H 2 O 0.01-0.10g / L; pH 6.5-7.5, 121°C
[0021] Sterilize under high pressure steam for 20min. The carbon source is selected from one or both of glucose, sucrose and maltose; the organic nitrogen source is selected from one or two of white corn steep liquor, peptone a...
Embodiment 2
[0028] Embodiment 2 Studies on thermal stability of Gibberella CA3-1
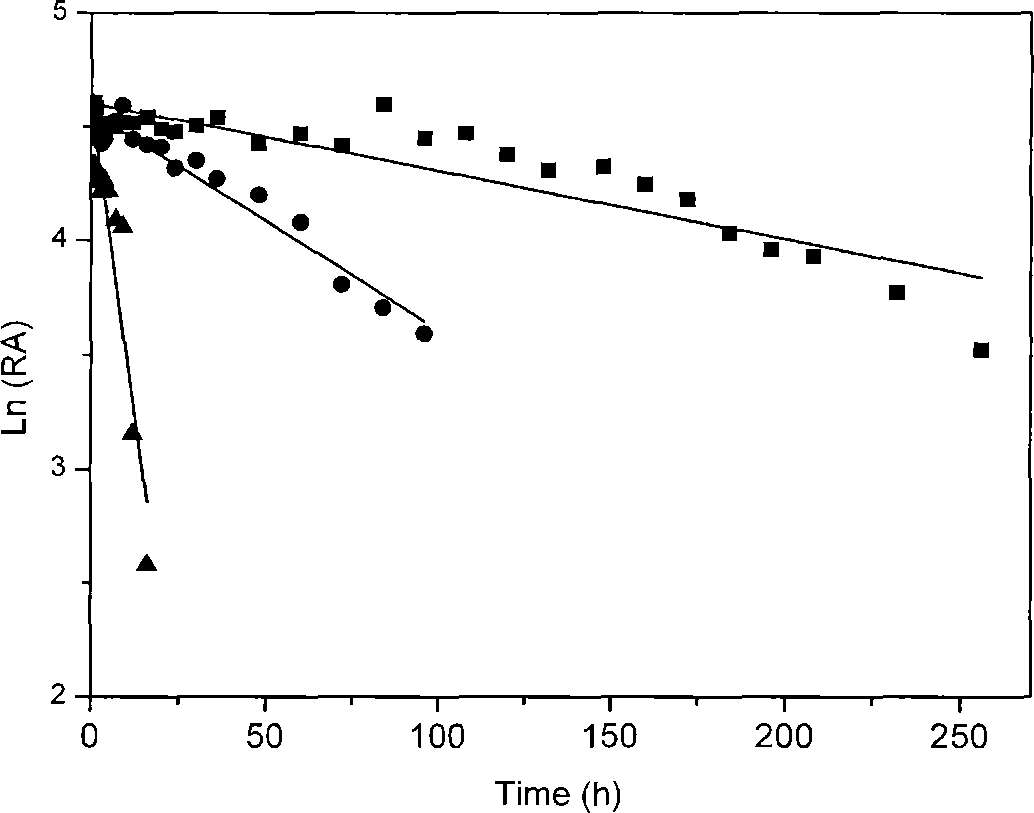
[0029] Take an appropriate amount of resting cell suspension, place them in water baths at 30°C, 40°C, and 50°C respectively, take samples regularly, convert 3-cyanopyridine according to the method described in Example 1, and use HPLC to track and detect the substrate The consumption and the production of the product are compared with the initial enzyme activity to calculate the relative enzyme activity, and the logarithm of the residual enzyme activity (lnRA) is plotted against time, and the slope K Decat That is, it represents the loss rate of enzyme activity, the results are shown in figure 2 .
Embodiment 3
[0030] The substrate spectrum research of embodiment 3 Gibberella CA3-1
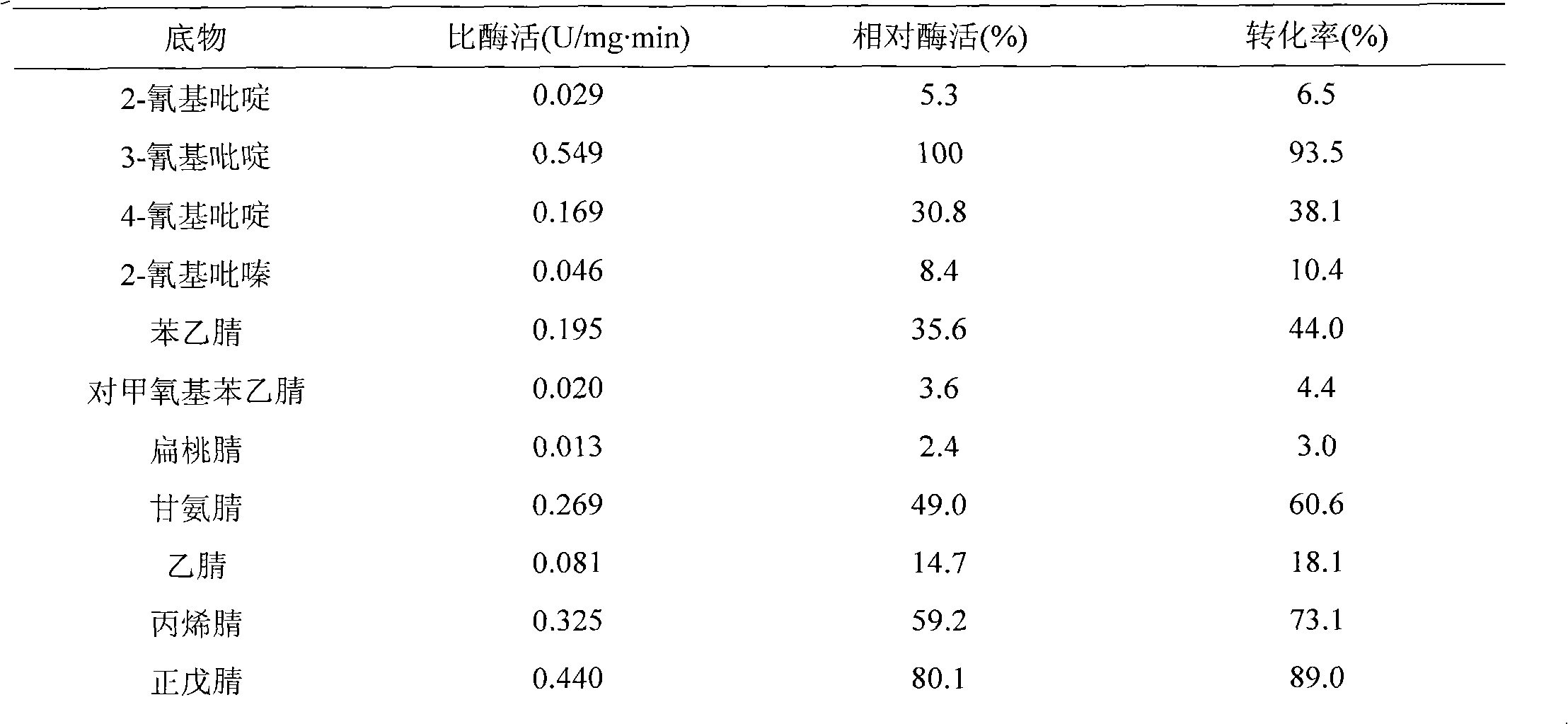
[0031] Get the resting cell of appropriate amount of Gibberella, add different nitrile compounds (final concentration 20mM) respectively, transform by the method in embodiment 2, the concentration of HPLC method detection substrate and product, calculate relative enzyme activity and conversion rate, The results are shown in the table below.
[0032] Table 1
[0033]
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com