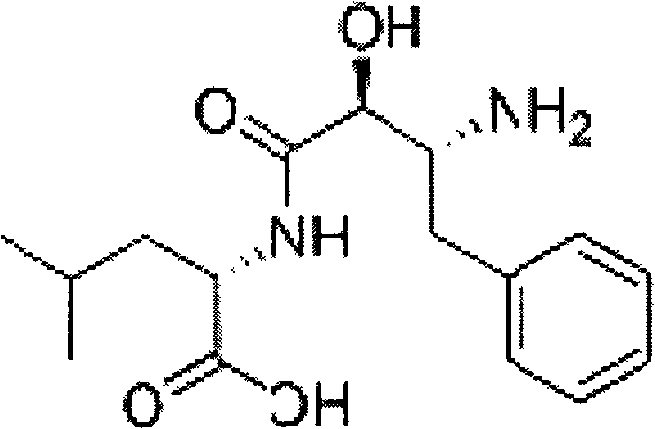
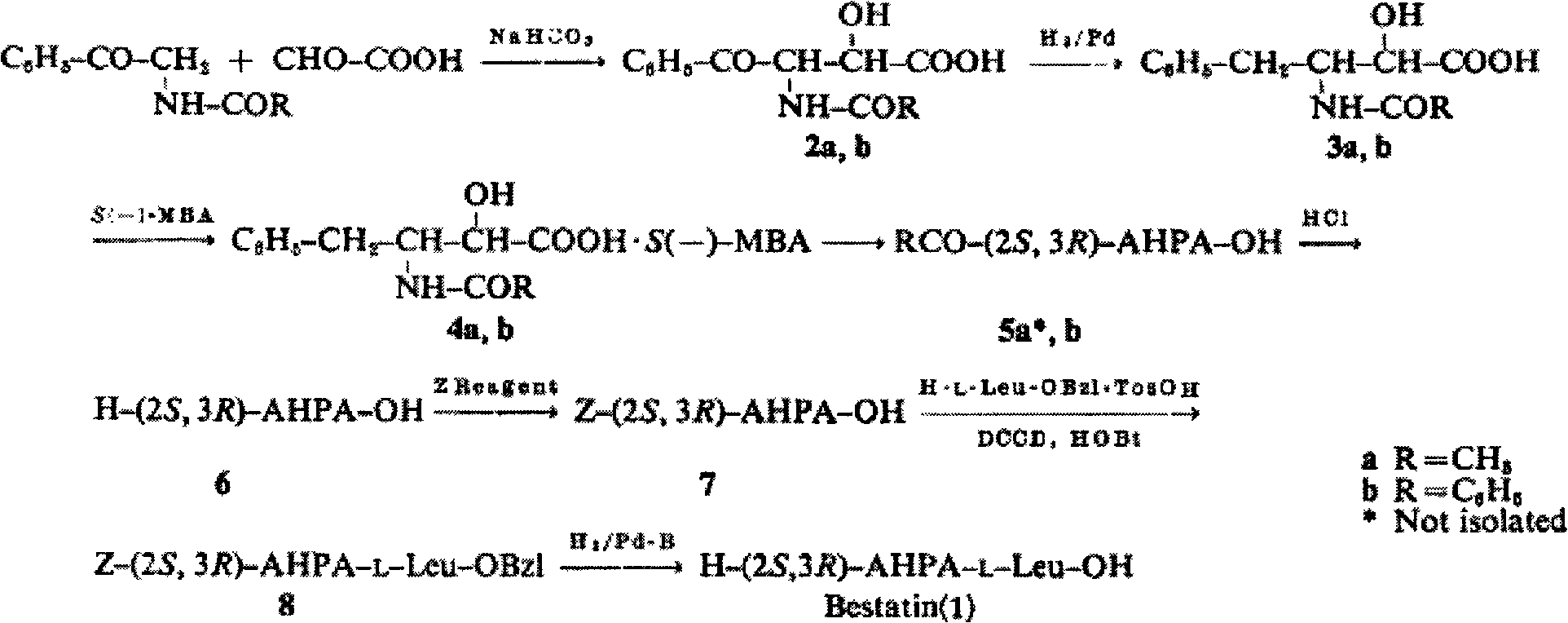Method for preparing ubenimex hydrolysis intermediates
A technology of Ubenimex and intermediates, which is applied in the field of preparation of Ubenimex hydrolysis intermediates, can solve the problems of wasting solvents, high energy consumption, and not suitable for large-scale production, and achieve energy saving, high yield, and Effect of saving solvent and energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
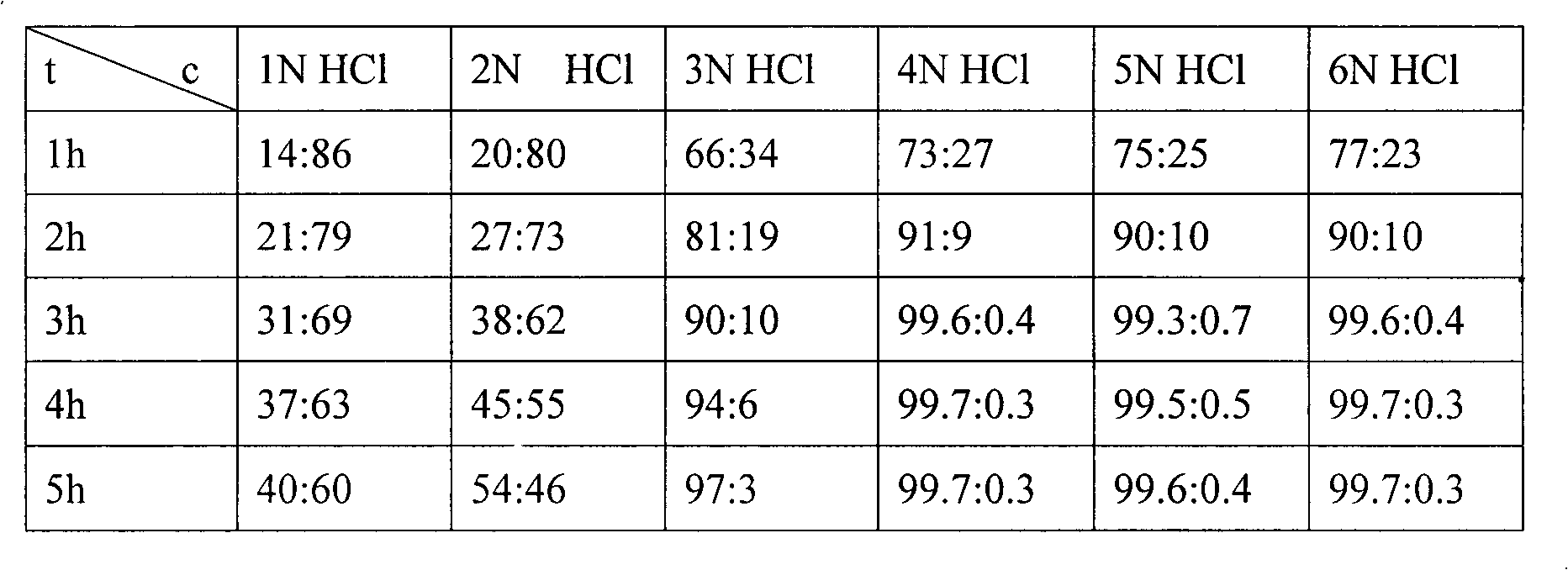
[0025] Put 200g of (2S, 3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid S(-)-α-phenethylamine salt into the reactor, add 640g of 4N hydrochloric acid aqueous solution, at 50°C Stir for 3 hours, add 4N sodium hydroxide solution to adjust pH=8.2, and filter with suction to obtain 109.6 g of crude product. The crude product was heated to 90°C with 986.4g of 60% DMF-water solution to dissolve, stirred and crystallized at room temperature, filtered with suction, and dried to obtain 103.63g of white crystals with a purity of 98.6% and an optical rotation of +31.7° (C=1, 1N HCl ), yield 93.27%.
Embodiment 2
[0027] Put 200g of (2S, 3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid S(-)-α-phenethylamine salt into the reactor, add 640g of 4N aqueous hydrochloric acid, at 70°C Stir for 3 hours, add 6N sodium hydroxide solution to adjust pH=8.2, and filter with suction to obtain 110.1 g of crude product. The crude product was heated to 90°C with 990.9g of 60% DMF-water solution to dissolve, stirred and crystallized at room temperature, filtered with suction, and dried to obtain 102.98g of white crystals with a purity of 98.5% and an optical rotation of +31.5° (C=1, 1N HCl), yield 92.87%.
Embodiment 3
[0029] Put 200g of (2S, 3R)-3-acetamido-2-hydroxy-4-phenylbutyric acid S(-)-α-phenethylamine salt into the reactor, add 1000g of 4N hydrochloric acid aqueous solution, at 60°C Stir for 3 hours, add 6N sodium hydroxide solution to adjust pH=8.2, and filter with suction to obtain 105.4 g of crude product. The crude product was heated to 90°C with 700g of 70% DMF-water solution to dissolve, stirred and crystallized at room temperature, suction filtered, and dried to obtain 103.50g of white crystals with a purity of 98.2% and an optical rotation of +31.4° (C=1, 1N HCl ), yield 93.21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com