Method for separating zirconium and hafnium
A technology of zirconium hydroxide and hafnium hydroxide, applied in the field of separation of zirconium and hafnium, can solve the problems of high water solubility of MIBK, poor working conditions, easy environmental pollution, etc. The effect of less mass transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for separating zirconium and hafnium, the specific steps are as follows:
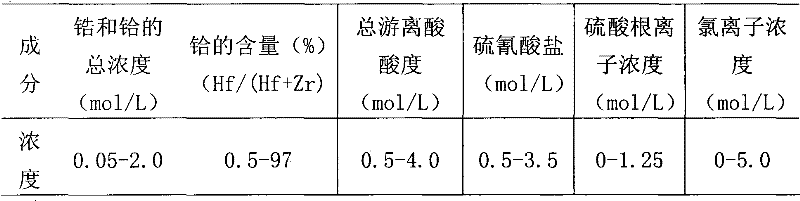
[0040] (1) Preparation of acidic feed liquid: the composition of the water phase is an initial total concentration of zirconium and hafnium ions of 1.3 mol / L, wherein the concentration of hafnium ions is about 0.016 mol / L, and the acidity of the water phase is 1.0 mol / L, [NH 4 SCN]=3.0mol / L, without adding (NH 4 ) 2 SO 4 .
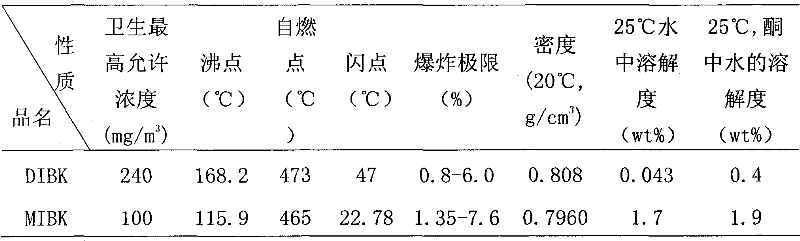
[0041] (2) extractant adopts DIBK, earlier the thiocyanic acid of extractant and equal volume 3.0mol / L is carried out pre-extraction once, then extractant is used as organic phase, feed liquid is used as water phase, control compares (organic phase: water Phase) is 2:1, the material liquid is subjected to single-stage extraction at room temperature, the mixing time of the two phases is 15 minutes, the zirconium is left in the water phase, and a hafnium-free zirconium solution is obtained, and then precipitated with ammonia water to obtain a zirconium hydroxide pre...
Embodiment 2
[0046] The organic phase is composed of 10% (v / v) TBP and 90% (v / v) DIBK and pre-extracted once with an equal volume of 1.0mol / L HSCN, and the aqueous phase is composed of an initial total concentration of zirconium and hafnium ions of 1.5mol / L L, where the concentration of hafnium ions is 0.018mol / L, the acidity of the aqueous phase is 1.0mol / L, [NH 4 SCN]=3.0mol / L, (NH 4 ) 2 SO 4 The addition amount is 0.8mol / L, the control ratio is 2:1, single-stage extraction is carried out at room temperature, the two-phase mixing time is 5 minutes, and ammonia water is used for precipitation to obtain a zirconium hydroxide precipitate and a loaded organic phase containing hafnium; The loaded organic phase was washed with 2.0mol / L hydrochloric acid and back-extracted with 12mol / L sulfuric acid, the ratio of washing and back-extraction was 1:2, and the mixing time of the two phases was 5 minutes to obtain 96% (Hf / (Hf +Zr)) rich hafnium solution, using ammonia for precipitation to obtain...
Embodiment 3
[0049] The organic phase is composed of 10% (v / v) TRPO and 90% (v / v) DIBK and pre-extracted once with an equal volume of 2.0mol / L HSCN, and the aqueous phase is composed of an initial total concentration of zirconium and hafnium ions of 2mol / L , where the concentration of hafnium ions is 0.02mol / L, the acidity of the aqueous phase is 0.5mol / L, [NH 4 SCN]=3.0mol / L, without adding (NH 4 ) 2 SO 4 , the control ratio is 2:1, single-stage extraction is carried out at room temperature, the two-phase mixing time is 10 minutes, and then precipitated with ammonia water to obtain a zirconium hydroxide precipitate and a loaded organic phase containing hafnium; the loaded organic phase is used 0.5mol / L hydrochloric acid for washing and 6.0mol / L sulfuric acid for back-extraction, the ratio of washing and back-extraction is 1:2, the two-phase mixing time is 10 minutes, to obtain 90% (Hf / (Hf+Zr)) rich The hafnium solution is precipitated by using ammonia water to obtain hafnium hydroxide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com